37 construct the octahedral crystal-field splitting diagram for the metal in each species.
Transcribed image text: Construct the octahedral crystal-field splitting diagram for the metal in each species, V(H_20)^3+_6 Fe(CN)^4-_6 FeCle^3-_6. Crystal Field Splitting in an Octahedral Field eg Energy 3/5 o o 2/5 o t2g e g - The higher energy set of orbitals (d z2 and d x2-y2) t 2g - The lower energy set of orbitals (d xy, d yz and d xz) Δ o or 10 Dq - The energy separation between the two levels The eThe eg orbitals are repelled by an amount of 0 6orbitals are repelled by an amount of 0.6 Δo The t2gorbitals to be stabilized to the ...
Construct the octahedral crystal field splitting diagram for the metal in each species. 3 for each species molecule or ion in the net ionic equation assign oxidation. A d1 octahedral complex is found to absorb visible light with the absorption maximum occcurring at 523 nm.
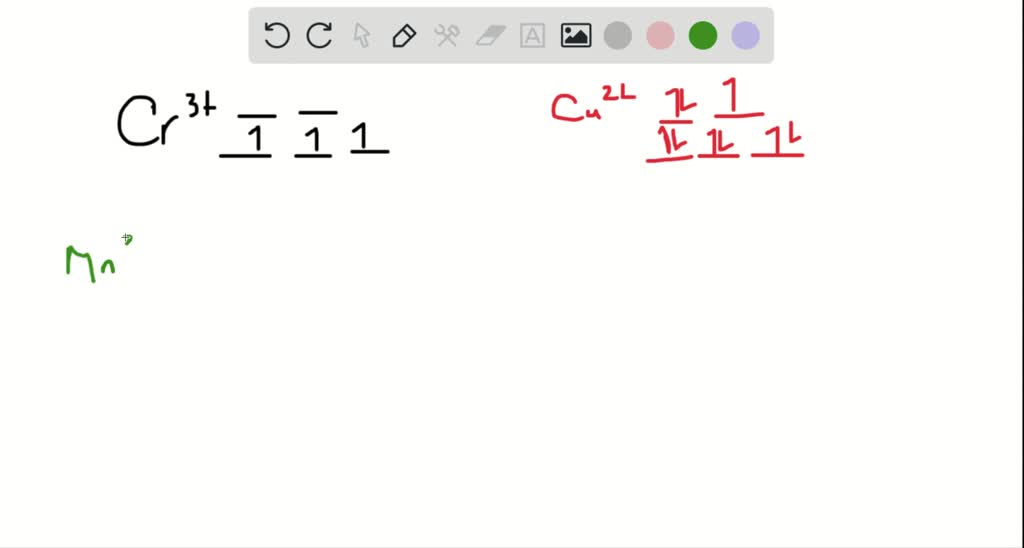
Construct the octahedral crystal-field splitting diagram for the metal in each species.
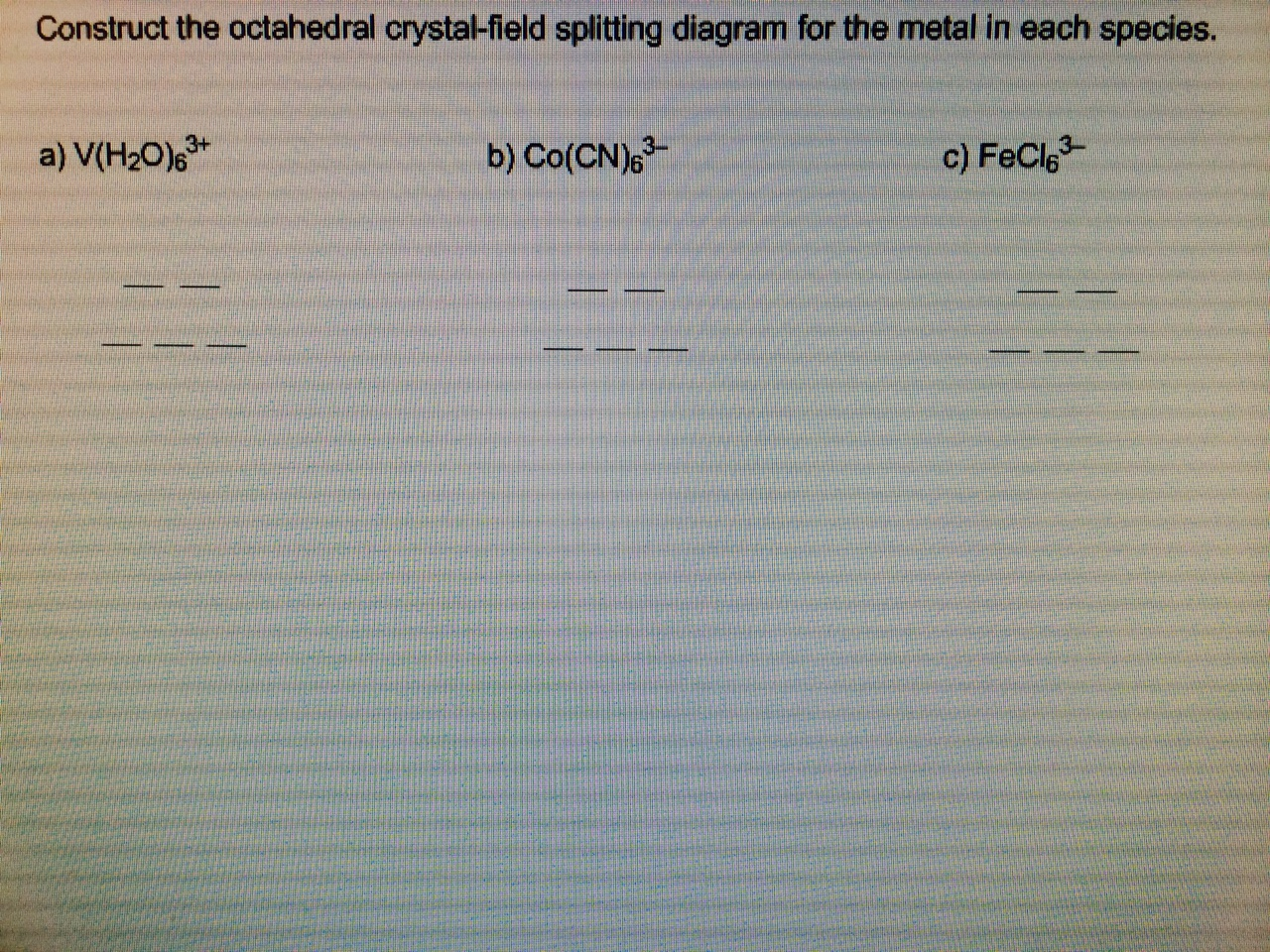
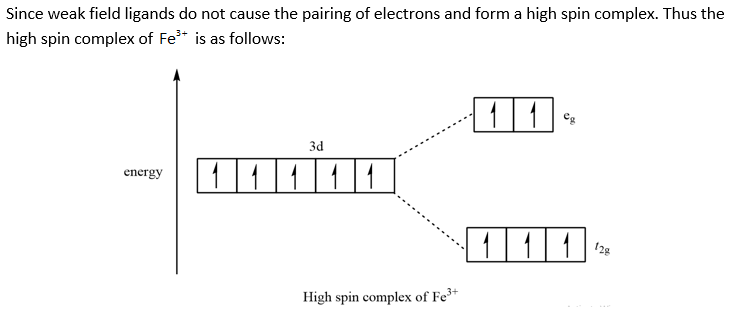
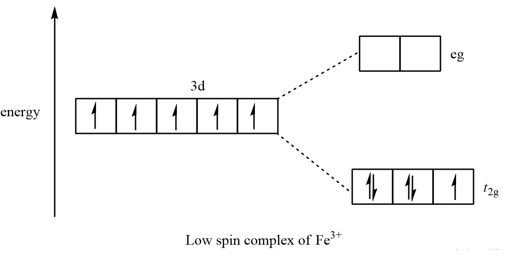
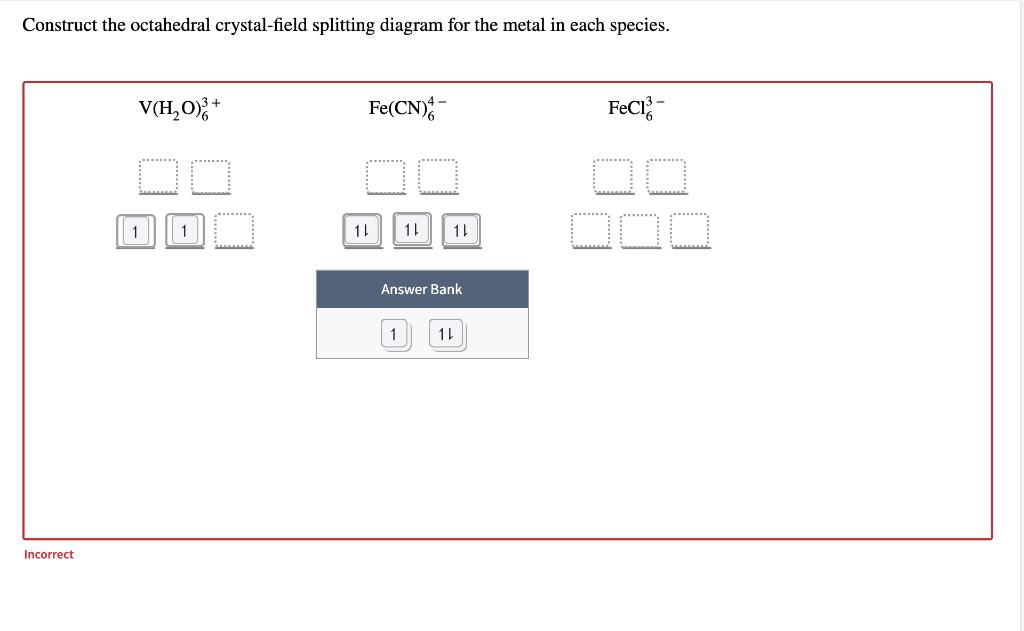
Academia.edu is a platform for academics to share research papers. Construct the octahedral crystal field splitting diagram for the metal in each species. Since the oxalate ligand is fairly low in the series a weak field ligand at this point you may not have studied ligand field theory yet which explains why it is a weak ligand. Cr4 mnh2o62 asked by katie on march 30 2012 chemistry based on crystal field ... Transcribed image text: Construct the octahedral crystal-field splitting diagram for the metal in each species, a) V(H_2O)_6^3+ Co(CN)_6^3- FeCl_6^3-.
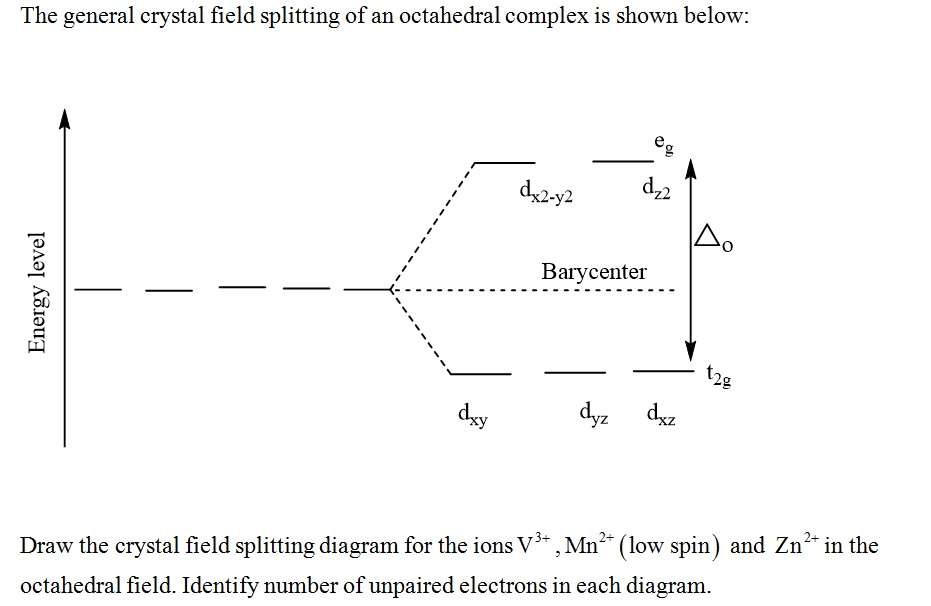
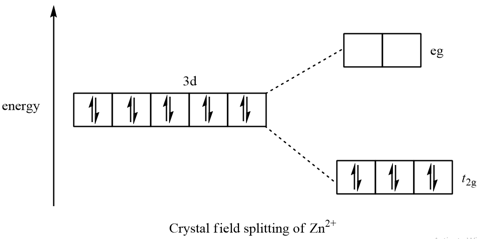
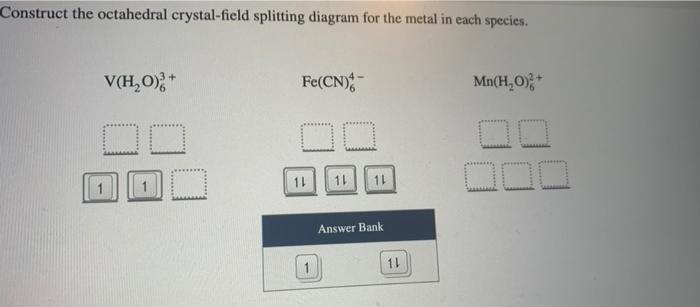
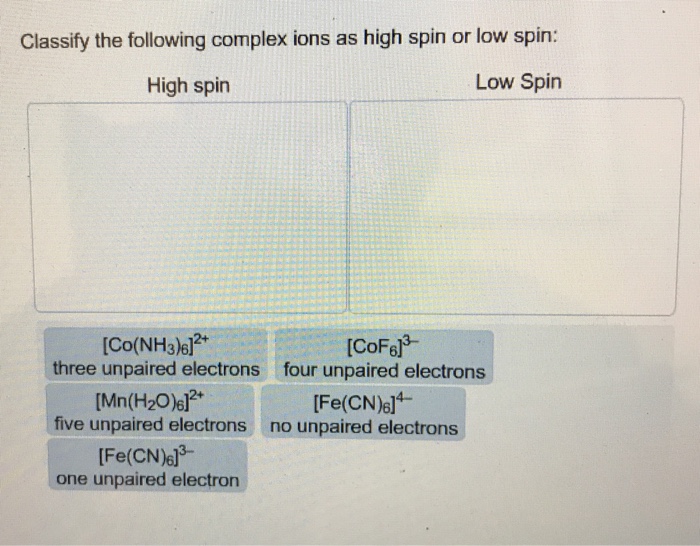
Construct the octahedral crystal-field splitting diagram for the metal in each species.. Answer to Construct the octahedral crystal-field splitting diagram for the metal in each species. V (H2O)63+ Co (CN)63 - Mn (H2O)62+. A d1 octahedral complex is found to absorb visible light, with the absorption maximum occcurring at nm. a) Calculate the crystal-field splitting energy, Δ, in. Transcribed image text: Construct the octahedral crystal-field splitting diagram for the metal in each species. V(H2O)63+ Co(CN)63 - Mn(H2O)62+. Construct the octahedral crystal field splitting diagram for the metal in each species. Lecture 9 crystal field theory for octahedral. Crystal Field Theory Chemistry Libretexts Cfse the stability that results from placing a transition metal ion in the crystal field generated by a set of ligands. how to construct the octahedral crystal-field splitting diagram for the metal in each species start by determining how many d electrons each metal species possesses. Make sure that degenerate orbitals obey Hund\'s rule of maximum multiplicity.
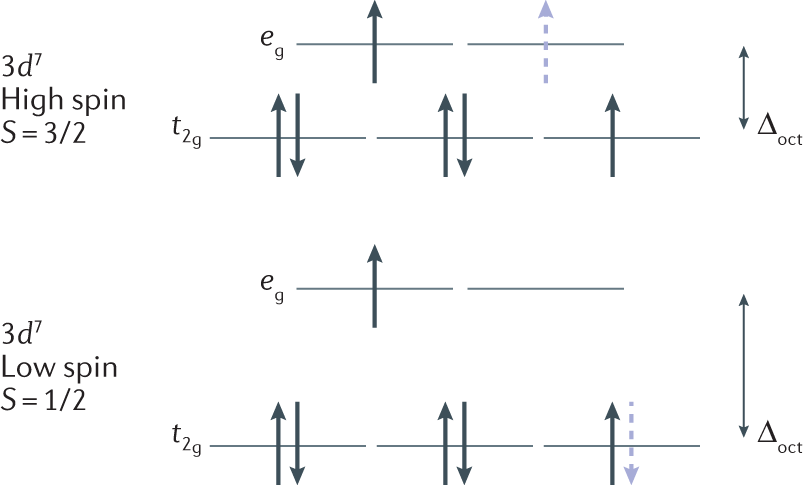
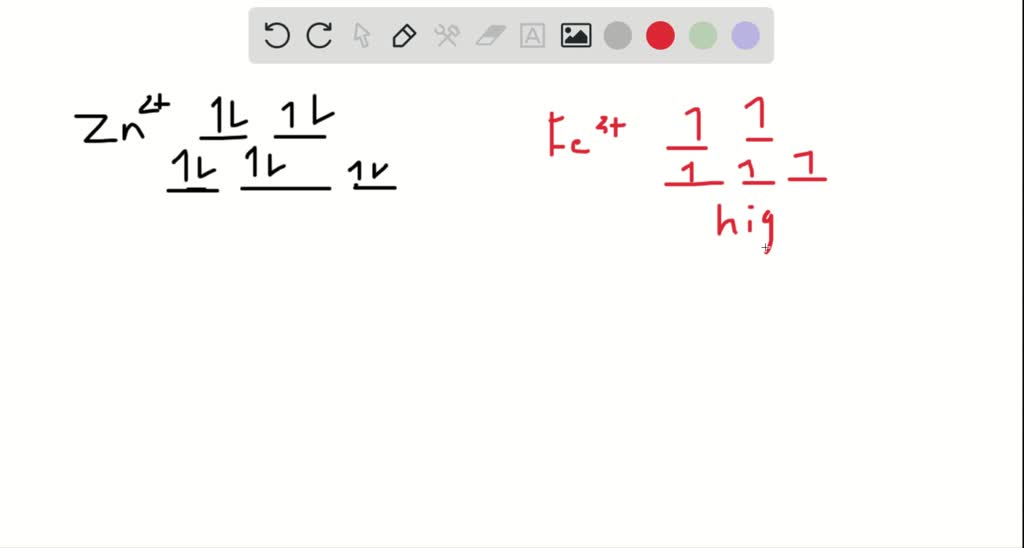
Question: Construct the octahedral crystal-field splitting diagram for the metal in each species. This problem has been solved! See the answerSee the ... Crystal field theory was established in 1929 treats the interaction of metal ion and ligand as a purely electrostatic phenomenon where the ligands are considered as point charges in the vicinity of the atomic orbitals of the central atom. Development and extension of crystal field theory taken into account the partly covalent nature of bonds ... FREE Expert Solution. Octahedral crystal-field splitting diagram → d-orbital electrons. high-spin - electrons can occupy the upper level (eg) low-spin - electrons can pair up with the electrons on the lower level (t2g) Recall that: weak field ligands → high spin → lowΔ or crystal field splitting energy values. Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Transcribed image text: Construct the octahedral crystal-field splitting diagram for the metal in each species. V (H2O)63+ Co (CN)63 - Mn (H2O)62+.
Transcribed image text: Construct the octahedral crystal-field splitting diagram for the metal in each species. V(H_2O)_6^3+ b) Fe(CN)_6^4- c) FeCl_6^3-. Transcribed image text: Construct the octahedral crystal-field splitting diagram for the metal in each species. a)V(H_2O)6^3+ b)Co(CN)6^3- c) FeCl_6^3-. This problem has been solved! See the answer. See the answer See the answer done loading. Construct the octahedral crystal-field splitting diagram for the metal in each species. V ( H 2 O ) 3 + 6 V (H2O)63+ Co ( CN ) 3 − 6 Co (CN)63− Mn ( H 2 O ) 2 + 6 Mn (H2O)62+ Construct the octahedral crystal-field splitting diagram for the metal in ... Academia.edu is a platform for academics to share research papers.

Solved Construct The Octahedral Crystal Field Splitting Diagram For The Metal In Each Species You Are Currently In A Labeling Module Turn Off Browse Mode Or Quick Nav Tab To Items Space Or Enter
Question: Construct the octahedral crystal-field splitting diagram for the metal in each species. V(H_2O)_6^3+ Co(CN)_6^3- Mn(H_2O)_6^2+ ...

Help With This Question Please Match The Appropriate Octahedral Crystal Field Splitting Diagram With The Given Spin Homeworklib
Transcribed image text: Construct the octahedral crystal-field splitting diagram for the metal in each species, a) V(H_2O)_6^3+ Co(CN)_6^3- FeCl_6^3-.
Construct the octahedral crystal field splitting diagram for the metal in each species. Since the oxalate ligand is fairly low in the series a weak field ligand at this point you may not have studied ligand field theory yet which explains why it is a weak ligand. Cr4 mnh2o62 asked by katie on march 30 2012 chemistry based on crystal field ...
Academia.edu is a platform for academics to share research papers.

Applications Of Nanomaterials In Asymmetric Photocatalysis Recent Progress Challenges And Opportunities Qiu 2021 Advanced Materials Wiley Online Library

Main Descriptors To Correlate Structures With The Performances Of Electrocatalysts Wang Angewandte Chemie International Edition Wiley Online Library

Molecules Free Full Text On The Origins Of Some Spectroscopic Properties Of Purple Iron The Tetraoxoferrate Vi Ion And Its Pourbaix Safe Space Html
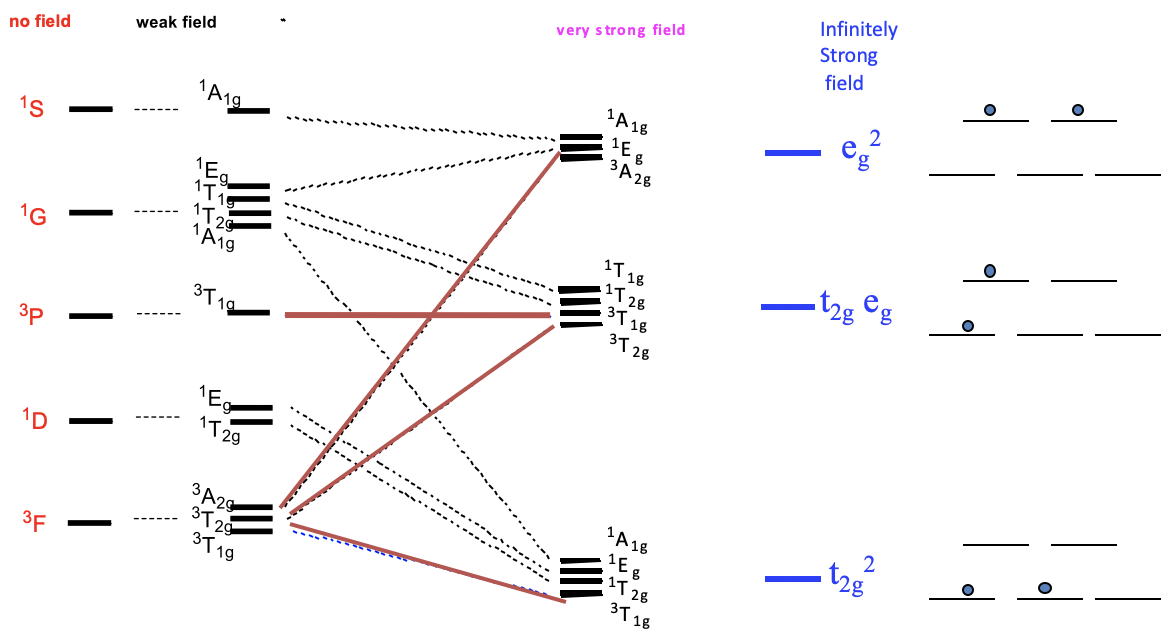
8 2 Term Splitting In Ligand Fields Selection Rules Tanabe Sugano Diagrams Metal To Ligand And Ligand To Metal Transitions Chemistry Libretexts

Porous Framework Based Hybrid Materials For Solar To Chemical Energy Conversion From Powder Photocatalysts To Photoelectrodes Inorganic Chemistry Frontiers Rsc Publishing Doi 10 1039 D1qi00543j

Construct The Octahedral Crystal Field Splitting Diagram For The Metal In Each Species Drivenheisenberg
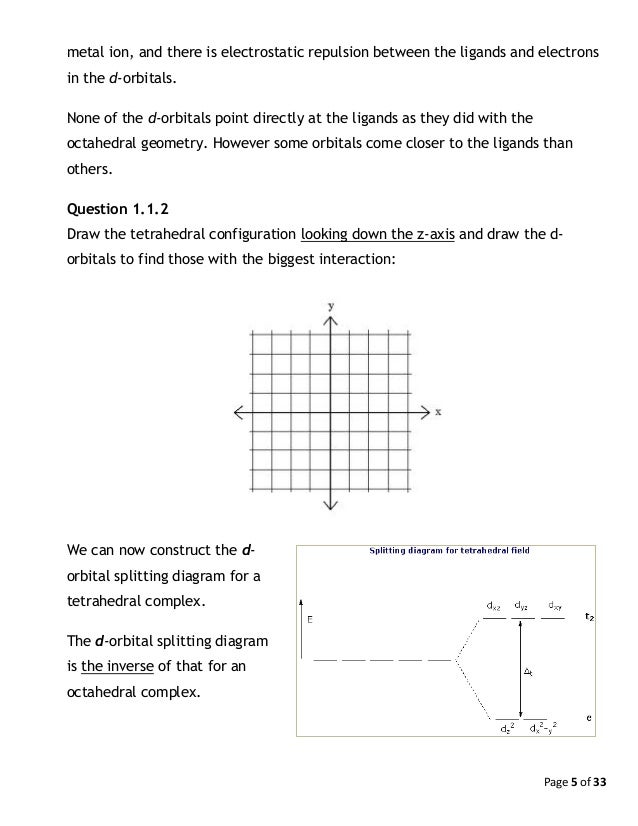
Construct The Octahedral Crystal Field Splitting Diagram For The Metal In Each Species Wiring Site Resource
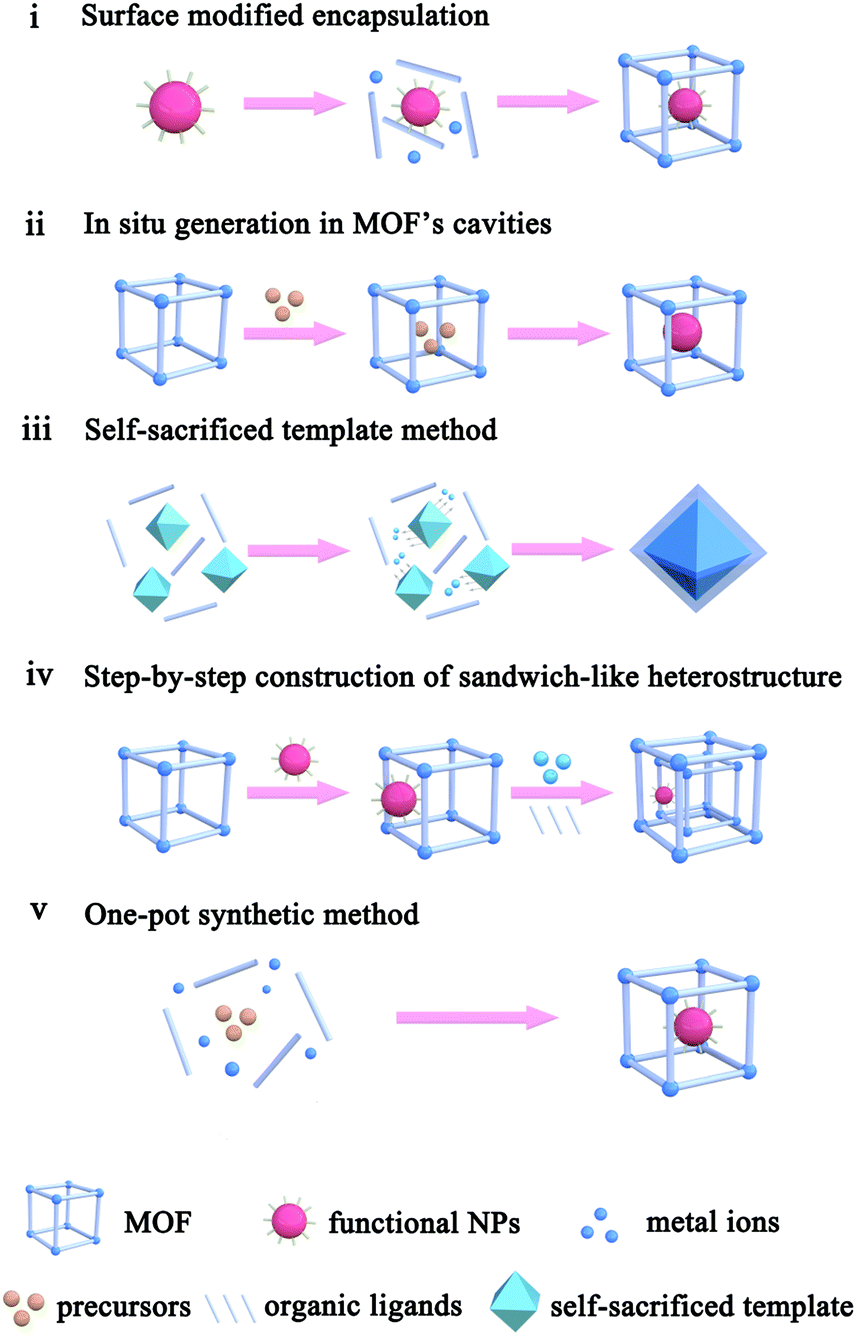
The Function Of Metal Organic Frameworks In The Application Of Mof Based Composites Nanoscale Advances Rsc Publishing

Recent Progress In Lanthanide Metal Organic Frameworks And Their Derivatives In Catalytic Applications Inorganic Chemistry Frontiers Rsc Publishing Doi 10 1039 D0qi01191f
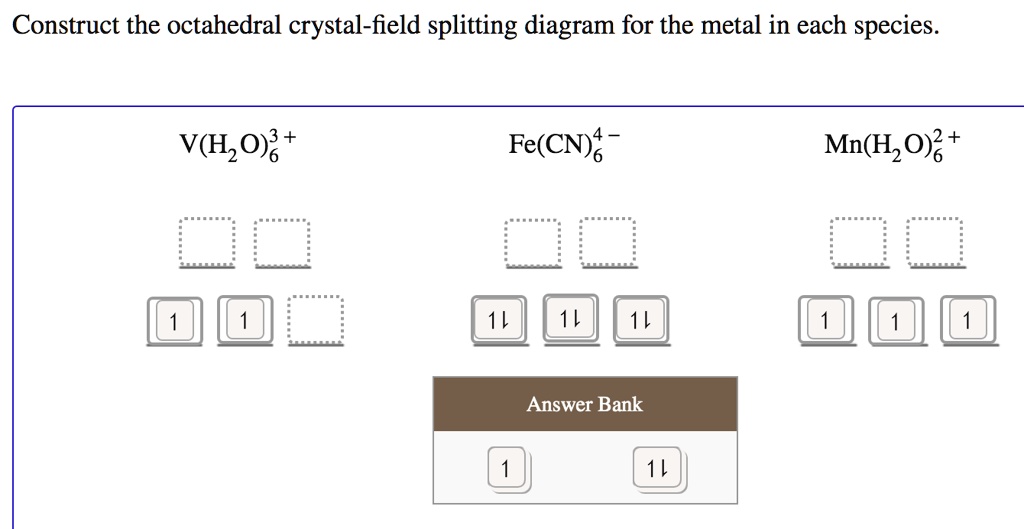
Solved Construct The Octahedral Crystal Field Splitting Diagram For The Metal In Each Species V H 0 Fe Cn G Mn Hz0 Answer Bank

A Theoretical Perspective On Charge Separation And Transfer In Metal Oxide Photocatalysts For Water Splitting Zhou 2019 Chemcatchem Wiley Online Library

A Review On Metal Organic Frameworks Photoelectrochemistry A Headlight For Future Applications Sciencedirect

Critical Review Of Perovskite Based Materials In Advanced Oxidation System For Wastewater Treatment Design Applications And Mechanisms Sciencedirect







0 Response to "37 construct the octahedral crystal-field splitting diagram for the metal in each species."
Post a Comment