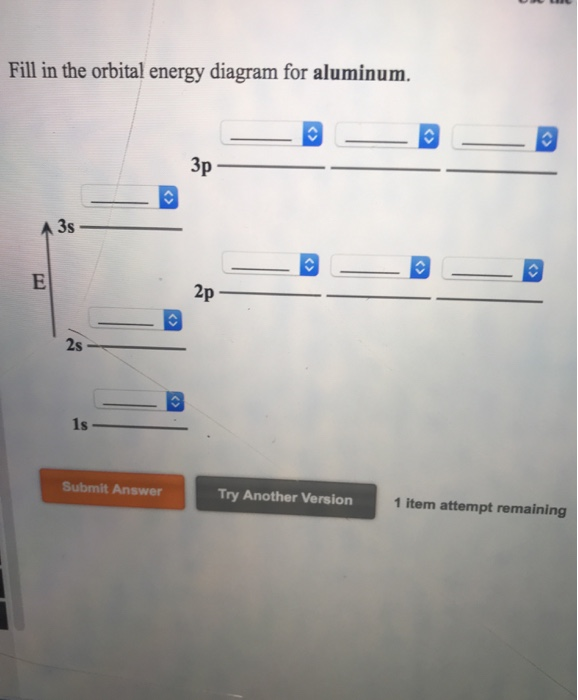
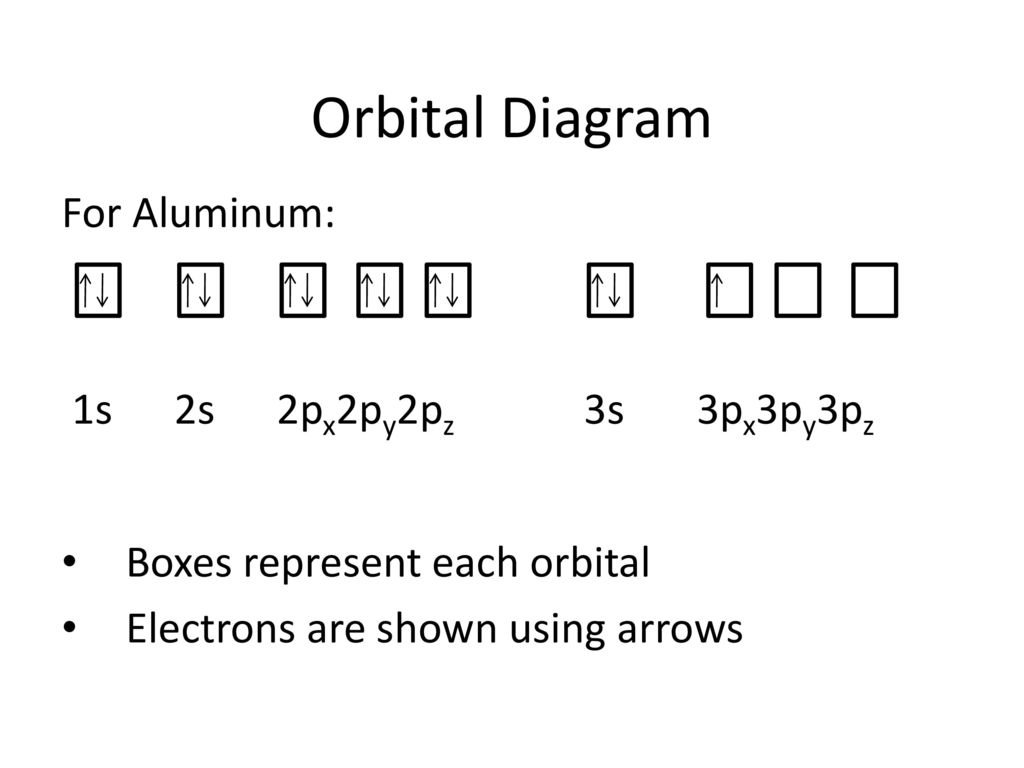
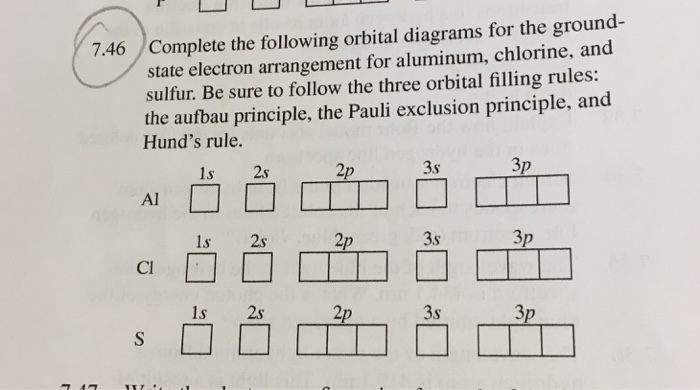
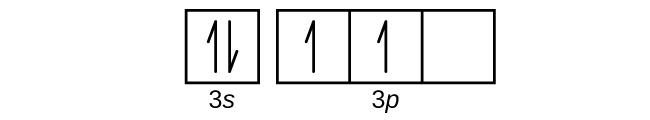
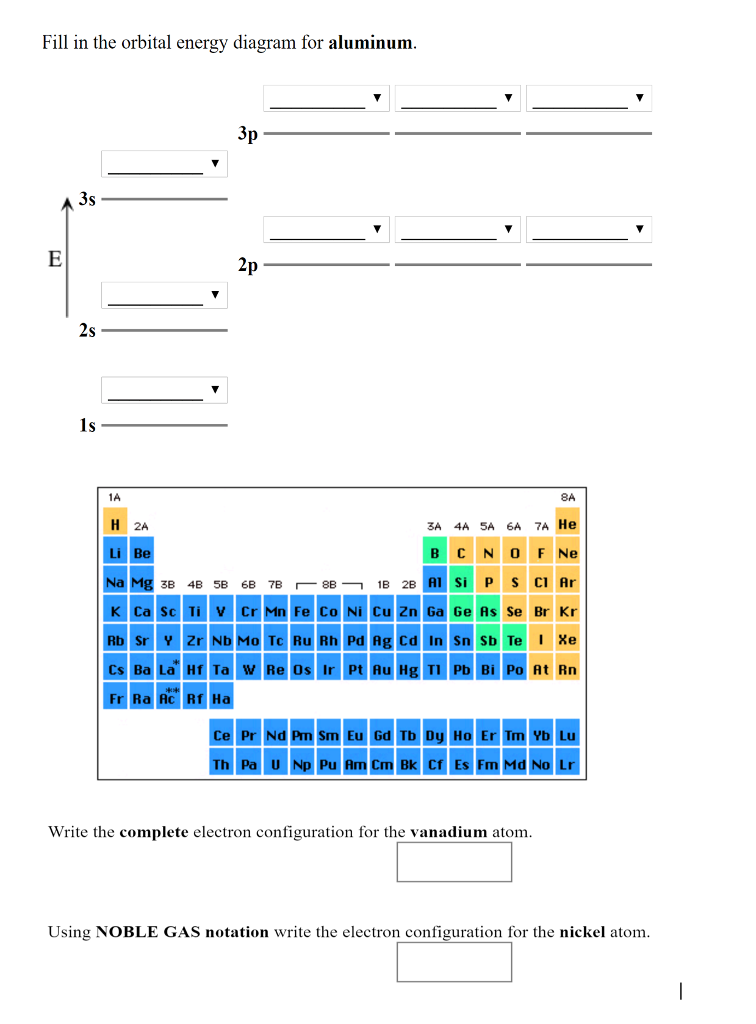
38 orbital diagram for aluminum
The order is summarized under the diagram. ... aluminum: 13: 1s 2 2s 2 2p 6 3s 2 3p 1: silicon: 14: 1s 2 2s 2 2p 6 3s 2 3p 2: phosphorus: 15: 1s 2 2s 2 2p 6 3s 2 3p 3: sulfur: 16: 1s 2 2s 2 2p 6 3s 2 3p 4: chlorine : 17: 1s 2 2s 2 2p 6 3s 2 3p 5: argon: 18: 1s 2 2s 2 2p 6 3s 2 3p 6: A. Box Diagrams of Electron Configuration If an atom has a partially filled sublevel, it may be important to ... 26 Jan 2021 — Aluminium Electron Configuration: Chemical element Aluminium can be written as AL and has atomic number 13. Aluminium is a soft, ductile, ...
Gabbard diagram of almost 300 pieces of debris from the disintegration of the five-month-old third stage of the Chinese Long March 4 booster on 11 March 2000. The trackers [clarification needed] who fed the database were aware of other objects in orbit, many of which were the result of in-orbit explosions. Some were deliberately caused during the 1960s anti-satellite weapon (ASAT) testing, and ...

Orbital diagram for aluminum
The orbital diagram will show one less e- for each halogen family and each noble gas family with full valence electron shell. A full shell is what the elements strive for so noble gas members require larger IE in order to lose their e-. 7. Where would the largest jump in ionization energies be for oxygen? (with the loss of how many electrons?) The largest ionization jump will occur following ... 23/09/2021 · The s orbital is spherical and can be occupied by a maximum of two electrons. Elements in column 1 have one electron in the s orbital, and elements … Electron Configuration of Aluminum — Aluminum's first two electrons fall in the 1s orbital, and the following two electrons go in the 2s orbital. The ...
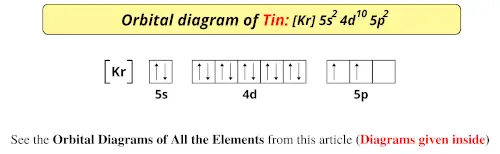
Orbital diagram for aluminum. 1 answerAluminum (Al) has only atomic orbitals. As a 3rd row element, however, it has a complete Ne electron configuration. One way to denote this is to write it as ... 3 Aug 2017 · 1 answerAluminum number 13 has 13 dots around it. Explanation: the electron configuration of Aluminum is 1s22S22p63s23p1. to draw the electron ... The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two electrons in the 3s. Since the 3s if now full we'll move ...24 Oct 2016 · Uploaded by Wayne Breslyn A solid solution describes a family of materials which have a range of compositions (e.g. A x B 1−x) and a single crystal structure.Many examples can be found in metallurgy, geology, and solid-state chemistry.The word "solution" is used to describe the intimate mixing of components at the atomic level and distinguishes these homogeneous materials from physical mixtures of components.
14/08/2020 · The 1s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. The energy increases as we move up to the 2s and then 2p, 3s, and 3p orbitals, showing that the increasing n value has more influence on energy than the increasing l value for small atoms. However, this pattern does not hold for larger atoms. The 3d orbital is higher in energy than the 4s orbital. Such ... Valence electrons can be counted using a Lewis electron dot diagram. In carbon dioxide, for example, each oxygen shares four electrons with the central carbon. These four electrons are counted in both the carbon octet and the oxygen octet because they are shared. Carbon dioxide: A Lewis dot diagram for carbon dioxide. Hydrogen and Lithium. However, many atoms below atomic number 20 often form ... Oxygen electron configuration is 1s 2 2s 2 2p 4.The period of oxygen is 2 and it is a p-block element. This article gives an idea about the electron configuration of oxygen(O) and orbital diagram, period and groups, valency and valence electrons of oxygen, bond formation, compound formation, application of different principles. The eighth element in the periodic table is oxygen. 1 answerWe are asked to give the full orbital diagram for aluminum (Al). Aluminum has 13 electrons to distribute because its atomic number is 13 and neutral atoms ...
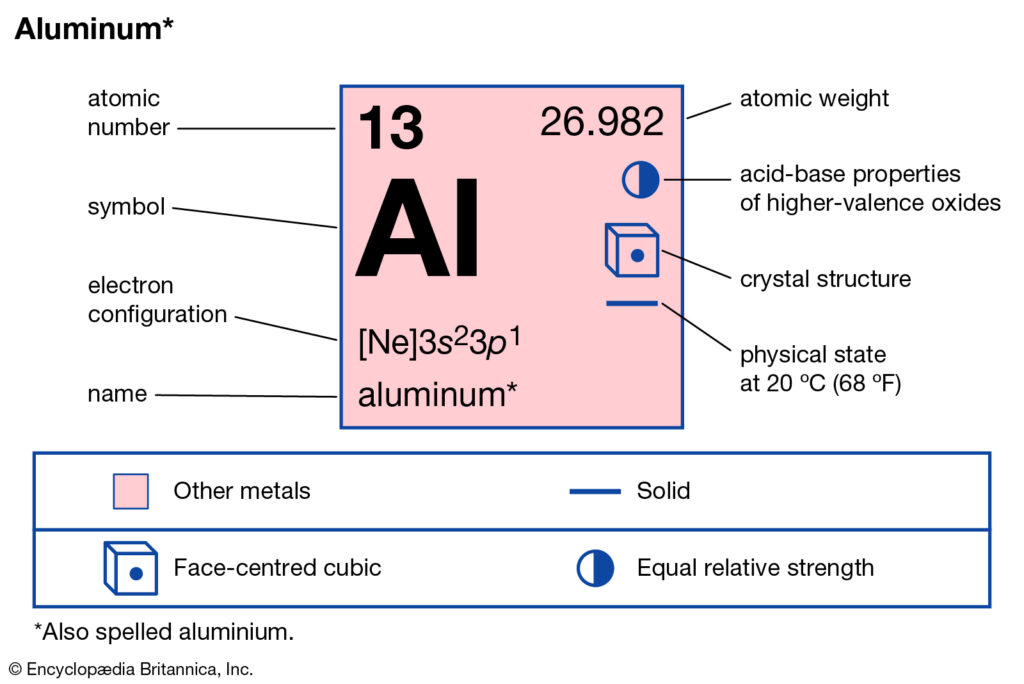
Aluminium (Al) has an atomic mass of 13. Find out about its chemical and ... Electron Configuration, [Ne] 3s2 3p1 ... Lewis Dot Diagram of Aluminium (Al). Electron Configuration of Aluminum — Aluminum's first two electrons fall in the 1s orbital, and the following two electrons go in the 2s orbital. The ... 23/09/2021 · The s orbital is spherical and can be occupied by a maximum of two electrons. Elements in column 1 have one electron in the s orbital, and elements … The orbital diagram will show one less e- for each halogen family and each noble gas family with full valence electron shell. A full shell is what the elements strive for so noble gas members require larger IE in order to lose their e-. 7. Where would the largest jump in ionization energies be for oxygen? (with the loss of how many electrons?) The largest ionization jump will occur following ...

Aufbau Principle Molecular Orbital Diagram Electron Configuration Bromine Atomic Orbital Others Png Pngwing

Electron Configurations How To Write Out The S P D F Electronic Arrangements Of Atoms Ions Periodic Table Oxidation States Using Orbital Notation Gce A Level Revision Notes
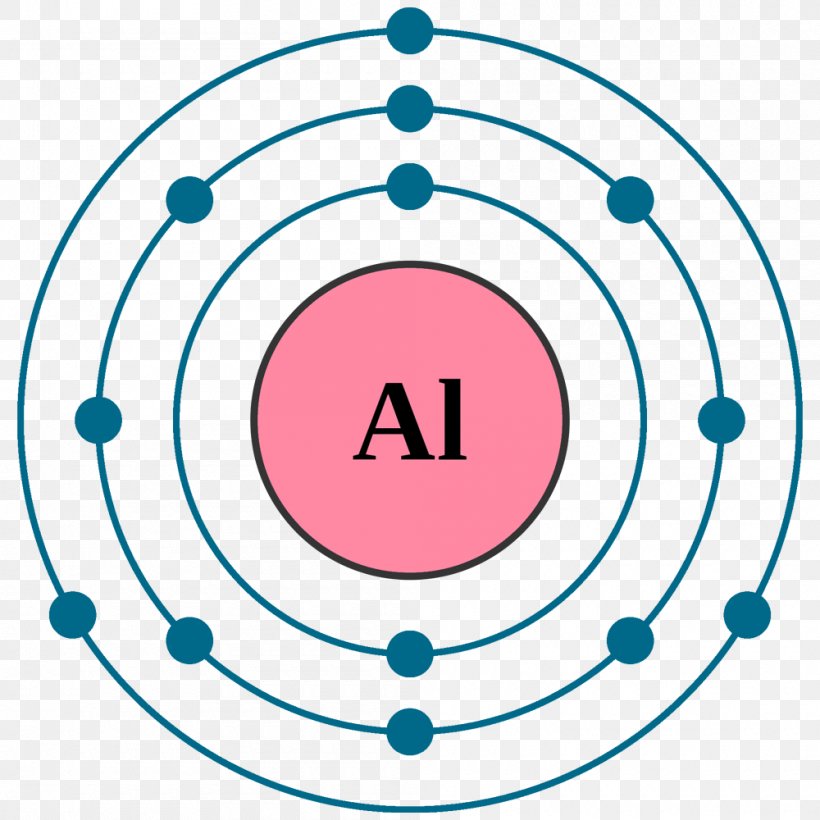
Atom Bohr Model Electron Configuration Chlorine Png 1000x1000px Atom Atomic Mass Atomic Number Atomic Theory Bohr

1 13al Konfigurasi Elektron Diagram Orbital Bilangan Kuantum Elektron Terakhir Konfigurasi Brainly Co Id


















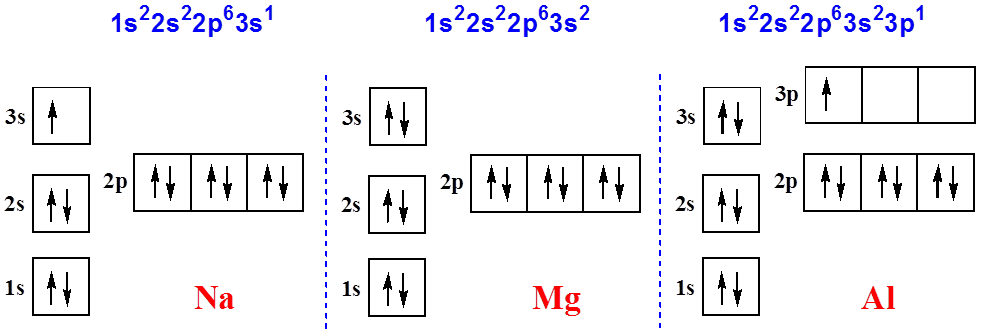




0 Response to "38 orbital diagram for aluminum"
Post a Comment