39 energy level diagram chemistry
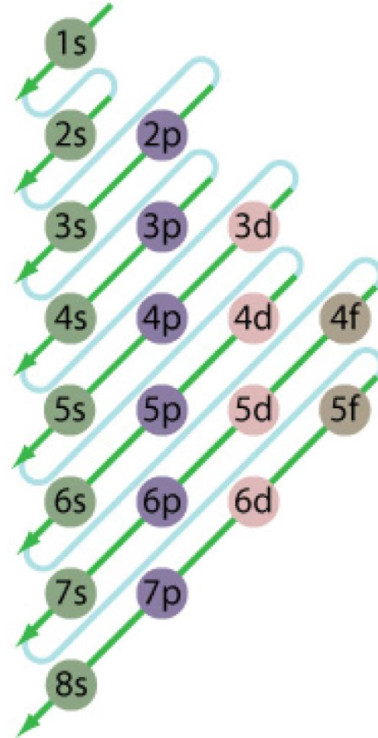
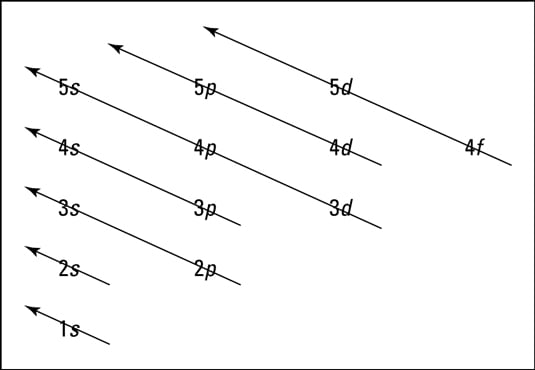
While Lewis diagrams and energy level structures can show connectivity and energy relationships of mol-ecules, they do not show the shape of the molecules. For this we need to picture atomic and molecular orbitals. l = 0 2. ATOMIC ORBITALS 2p x 2p y 2p z l = 1 x y z n = 2 This is an accurate representation of a 2p x orbital. This is a common ... Chemistry 2017. DAY ONE: October 3 ... •In each energy level, electrons fill sublevels in a certain order Level 1: a) only has one s sublevel (a spherical shape) ... diagram. Order of Orbitals—Periodic Table For the d block n-1 For the f block n-2. Hotel analogy video s s s s p d d p p f These are 4 levels each
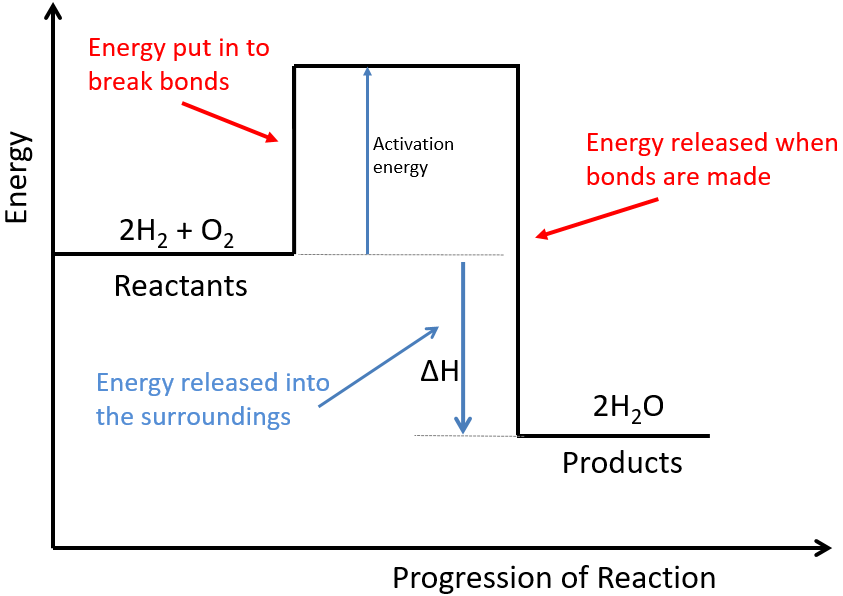
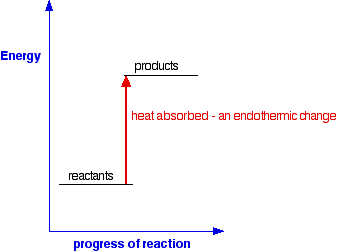
An energy level diagram is a diagram that shows the energies of the reactants, the transition state(s) and the products of the reaction with time The transition state is a stage during the reaction at which chemical bonds are partially broken and formed
Energy level diagram chemistry
The energy level diagram gives us a way to show what energy the electron has without having to draw an atom with a bunch of circles all the time. Let's say our pretend atom has electron energy levels of zero eV, four eV, six eV, and seven eV. Note that moving left or right on an energy level diagram doesn't actually represent anything ... Chemistry; Chemistry questions and answers; This energy diagram shows the allowed energy levels of an electron in a certain atom. (Note: the SI prefix 'zepto' means 10-21. You can find the meaning of any SI prefix in the ALEKS Data tab.) 1400 1200 P 1000 ol. energy (z)) -В -A Use this diagram to complete the table below. Chemistry Lesson 3.2Energy Level DiagramsPauli Exclusion PrincipleHund's RuleAufbau PrincipleDiagonal RulePractice problems#energyLevelDiagram #quantum #ketz...
Energy level diagram chemistry. In this video we explained how to make Energy Level Diagram of molecules (H2) and how to find stability, Bond Order, Magnetic Properties of H2 molecule. #che... The molecular energy levels are labelled by the molecular term symbols. The specific energies of these components vary with the specific energy state and the substance. Energy level diagrams. There are various types of energy level diagrams for bonds between atoms in a molecule. Examples Energy level diagrams (sometimes called reaction profiles) are graphical representations of the relative energies of the reactants and products in chemical reactions. The energy of the reactants and products are displayed on the y-axis and the reaction pathway is shown on the x-axis. Arrows on the diagrams indicate whether the reaction is exothermic ( downwards pointing) or endothermic ( upwards pointing) An orbital energy level diagram (or just orbital diagram) shows the relative energies of orbitals and how electrons are distributed among orbitals within a subshell. In an orbital energy level diagram, individual orbitals are usually represented by horizontal lines whose vertical position conveys the qualitative relative energies of the orbitals. The electrons are represented as arrows with the direction of the arrow communicating the sign of ms (the ↑ arrow represents ms = +½ and the ...
Diagram representing the arrangement of orbitals in order of their increasing energies are called energy level diagrams. Important observations from energy level diagrams of multi electron atoms are: 1)The sub shell of a particular shell do not have equal energies.For Ex: 2s and 2p have different energies. In this video we take a look at energy level diagrams and how we can interpret them. They allow us to see visually whether a reaction is endothermic or exoth... Enthalpy level diagrams. When a Hess' law energy cycle is presented graphically showing the relative chemical energies of the reactants and products, this is called an energy, or enthalpy level diagram. Personally, I find these more logical than Hess' energy cycles, however it's a matter of individual preference. Join our community below for all the latest videos and tutorials!Website - https://thenewboston.com/Discord - https://discord.gg/thenewbostonGitHub - https:/...
The electrons surrounding an atom are located in regions around the nucleus called “energy levels”. An energy level represents the 3-dimensional space surrounding the nucleus where electrons are most likely to be. The first energy level is closest to the nucleus. The second energy level is a little farther away than the first. This is the final result: It is quite easy to use, on the GitHub readme there is a small tutorial. You have to create an instance of ED class and then add the energy levels to it. from energydiagram import ED diagram = ED () diagram.add_level (0,'Separated Reactants') diagram.add_level (-5.4,'mlC1') diagram.add_level (-15.6,'mlC2','last ... Chemistry; Chemistry questions and answers; This energy diagram shows the allowed energy levels of an electron in a certain atom. (Note: the SI prefix 'zepto' means 10-21. You can find the meaning of any SI prefix in the ALEKS Data tab.) 1400 1200 圖 1000 ol energy (z)) -B Use this diagram to complete the table below. This is the first time I reblog a post from a fellow computational chemist and the reason why I do it is because of its beautiful simplicity and usefulness. Given the scope this blog has taken I think this post becomes most appropriate. This post will show you how to create an energy level diagram using nothing but MS Excel.

3 14 Triple Only Draw And Explain Reaction Profile Diagrams Showing Dh And Activation Energy Tutormyself Chemistry
The energy level diagram is therefore as shown below. Note that the total pi electron energy is more negative (more bonding) than the two electrons of the ethene pi bond, by 0.82b. This is the delocalization energy, i.e., the additional stabilization of these two electrons derived from their delocalization over three atoms, instead of just two.
Students will use data from the lab to model and explain the phenomena using energy level diagrams. For the task that follows, students will be given temperature data from a chemical reaction and, based on these data, students relate the change in temperature to the energy associated with chemical reactions in order to determine whether a reaction is exothermic or endothermic.
An energy level diagram is a diagram that shows the energies of the reactants, the transition state(s) and the products of the reaction as the reaction proceeds ( called 'extent of the reaction' below)
Energy level diagrams. are used to model energy. changes during reactions. They show the relative energy levels of the products and reactants . Exothermic reaction
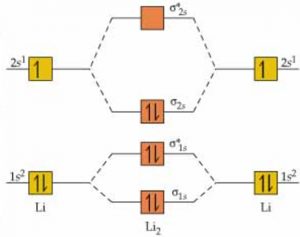
Energy Level Diagram For Molecular Orbitals Chemical Bonding And Molecular Structure Chemistry Class 11
Rates of Reaction. What are Energy Level Diagrams?. The change in energy of a chemical reaction can be plotted against its progress as the reactants turn into products.The energy level diagram for an exothermic reaction is shown below.. Going from the reactants to the top of the curve, we are going up the energy axis of the graph. Energy is being put in to break bonds in the reactants.
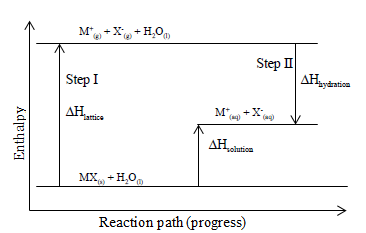
The energy levels are tracked using an enthalpy diagram. An enthalpy diagram plots information about a chemical reaction such as the starting energy level, how much energy needs to be added to ...
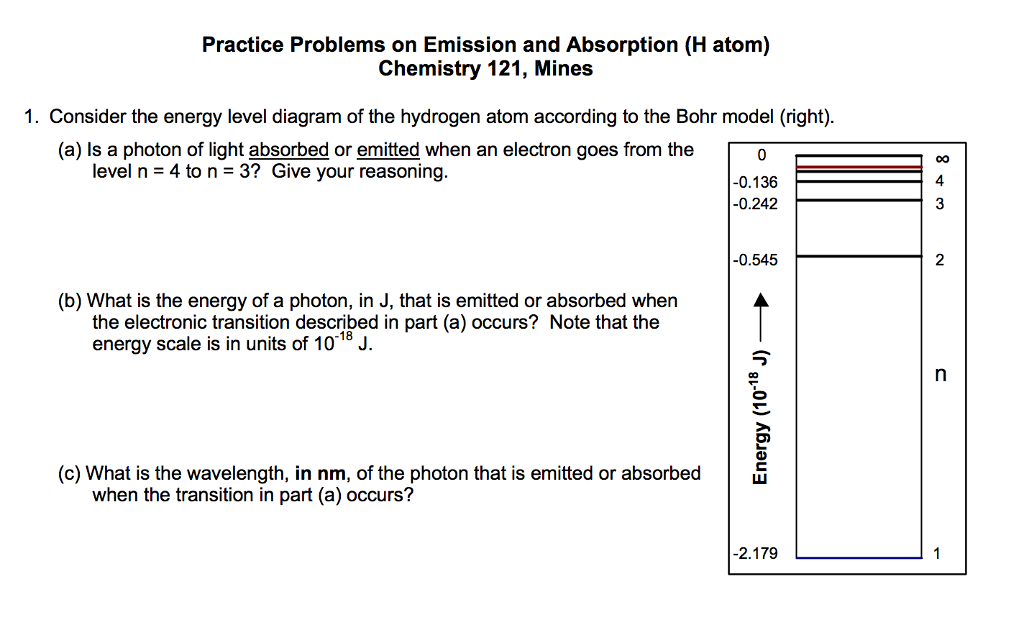
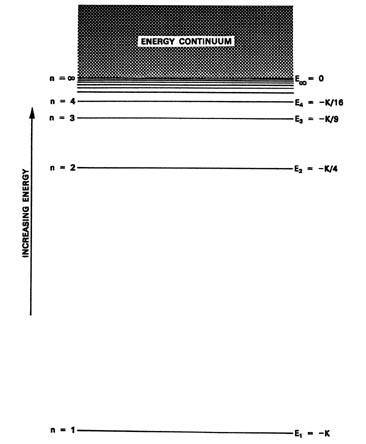
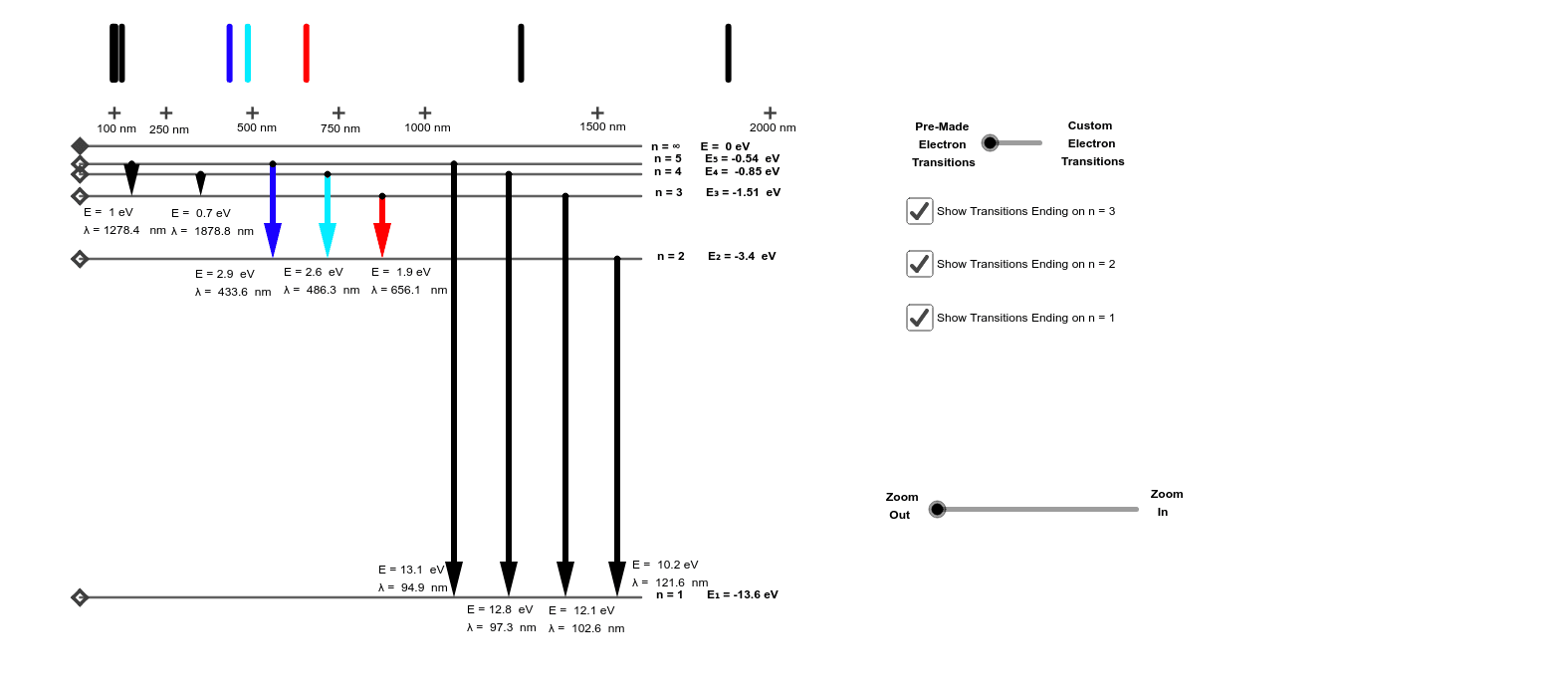
Re: H-Atom Energy Level Diagram. Hi! The energy of electrons can only have certain values. This corresponds to the energy levels n=1, n=2, n=3, etc. When an electron absorbs a specific wavelength of light that matches the energy needed to move, it can jump levels. An electron at a higher level with high energy will eventually drop back to a ...
Define activation energy and label it on a diagram. Explain why reactions are endothermic or exothermic overall. This lesson, students will draw energy level diagrams for exothermic and endothermic reactions. Students will identify activation energy and overall energy change in energy diagrams. We will also explain why reactions are endothermic ...
Filed Under: Chemical Bonding and Molecular Structure, Chemistry, Class 11 Tagged With: bond order, Energy level diagram for Molecular orbitals About Mrs Shilpi Nagpal Author of this website, Mrs Shilpi Nagpal is MSc (Hons, Chemistry) and BSc (Hons, Chemistry) from Delhi University, B.Ed (I. P. University) and has many years of experience in ...
Energy level diagram of Nitrogen and Oxygenhttps://www.klasspm.com/engineering/jntuh_1/btech_5/civil-and-environmental-engineering_53/1-year-i-semester_28/en...
An energy level diagram is a diagram that shows the energies of the reactants, the transition state(s) and the products of the reaction with time; The transition state is a stage during the reaction at which chemical bonds are partially broken and formed
What is energy level diagram? In chemistry, an electron shell, or energy level, may be imagined as an orbit with electrons around the nucleus of an atom. The closest shell to the nucleus is called the “K shell” followed by the “L shell” then the “M shell” and so on away from the nucleus. The shells can be denoted by alphabets (K, L, M, …) or quantum numbers ( n = 1, 2, 3, 4 and so on).
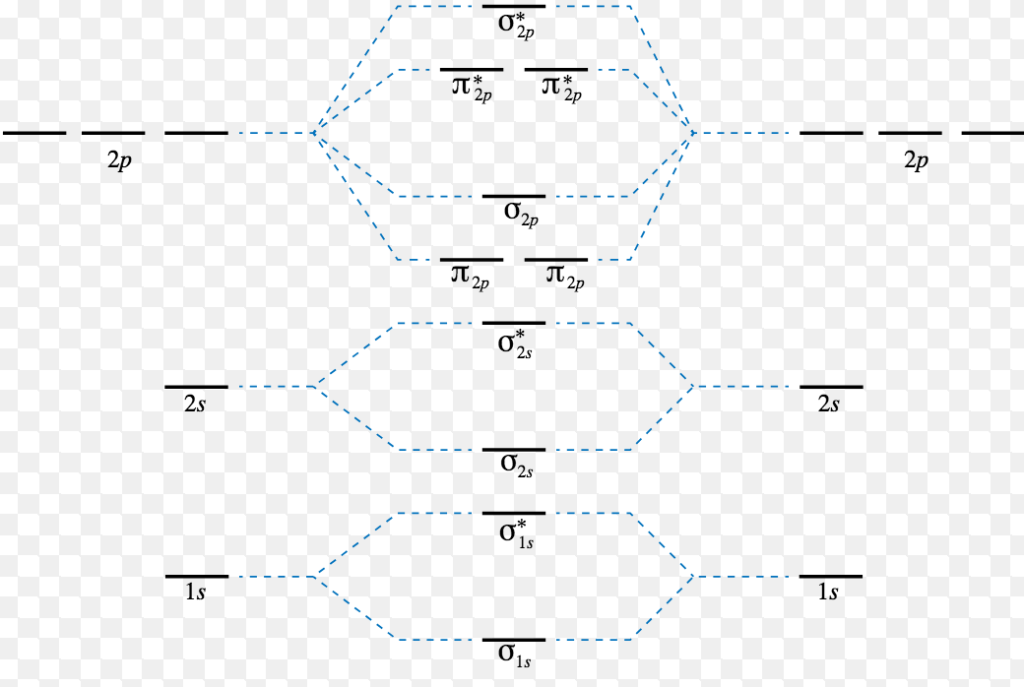
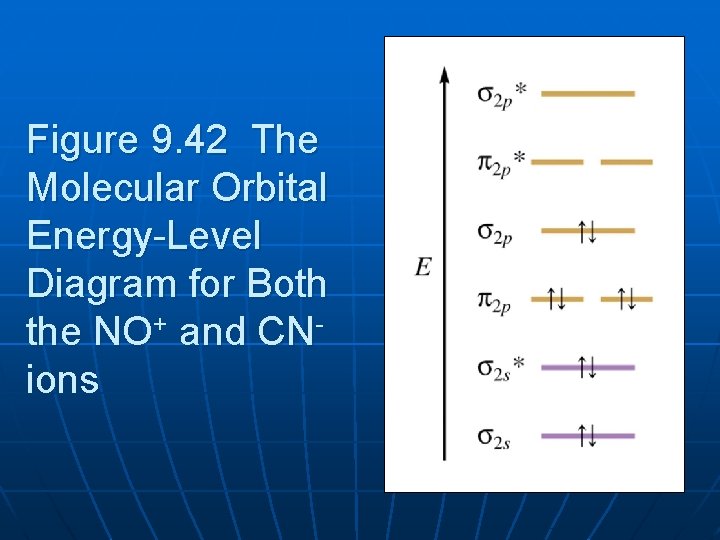
energy-level diagram for the NO molecule. We assume that orbital order is the same as that for N2. The bond order is 2.5. Figure 9.42: The molecular orbital energy-level diagram for both the NO+ and CN-ions. Figure 9.43: A partial molecular orbital energy-level diagram for the HF molecule.
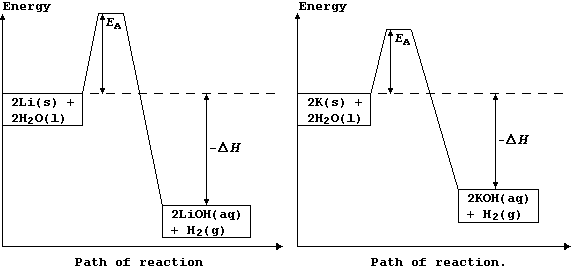
1 Chemistry Principles (a) States of matter ... The change in heat (enthalpy change) can be represented on an energy level diagram. ΔH must also labelled. In an exothermic reaction, the reactants have more energy than the products. Energy is given out in the form of heat which warms the surroundings.

Energy Level Diagram Of The Relevant States Of 11 Bn Energy Level Transparent Png 724x515 Free Download On Nicepng
Worked example: Drawing energy level diagrams of the combustion of methane. Answer. Step 1: The chemical equation for the complete combustion of methane is: CH4 (g) + 2O2 (g) → CO2 (g) + 2H2O (l) Step 2: Combustion reactions are always exothermic (Δ H is negative) so the reactants should be drawn higher in energy than the products.
3 4. What is the effect of 2s‐2p mixing on the energy level diagram? The 2px provides bonding (constructive) interactions in the s2s and s2s* m.o.’s LOWERING their energies. The 2s participates in the s2p and s2p* m.o.’s with antibonding (destructive) interactions and thus RAISES the energies of the s2p and s2p* m.o.’s.
Chemistry Lesson 3.2Energy Level DiagramsPauli Exclusion PrincipleHund's RuleAufbau PrincipleDiagonal RulePractice problems#energyLevelDiagram #quantum #ketz...
Chemistry; Chemistry questions and answers; This energy diagram shows the allowed energy levels of an electron in a certain atom. (Note: the SI prefix 'zepto' means 10-21. You can find the meaning of any SI prefix in the ALEKS Data tab.) 1400 1200 P 1000 ol. energy (z)) -В -A Use this diagram to complete the table below.
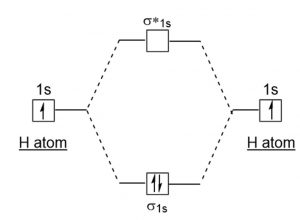
Energy Level Diagram For Molecular Orbitals Chemical Bonding And Molecular Structure Chemistry Class 11
The energy level diagram gives us a way to show what energy the electron has without having to draw an atom with a bunch of circles all the time. Let's say our pretend atom has electron energy levels of zero eV, four eV, six eV, and seven eV. Note that moving left or right on an energy level diagram doesn't actually represent anything ...
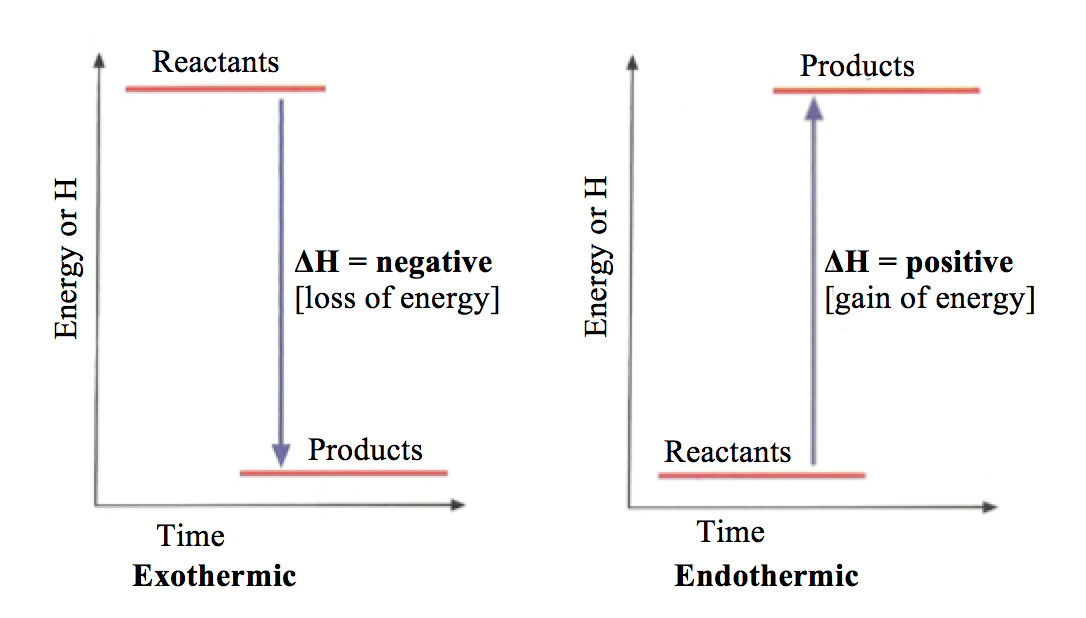
4 14 Represent Exothermic And Endothermic Reactions On A Simple Energy Level Diagram Igcse Chemistry Revision Help
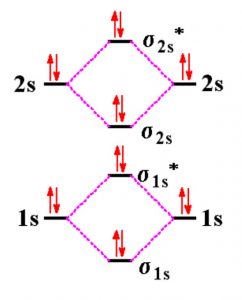
Energy Level Diagram For Molecular Orbitals Chemical Bonding And Molecular Structure Chemistry Class 11
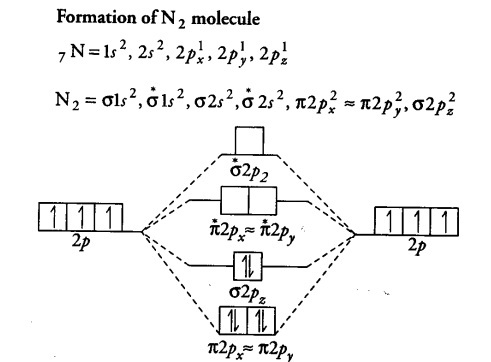
Use The Molecular Orbital Energy Level Diagram To Show That Cbse Class 11 Chemistry Learn Cbse Forum

Igcse Chemistry 4 14 Represent Exothermic And Endothermic Reactions On A Simple Energy Level Diagram

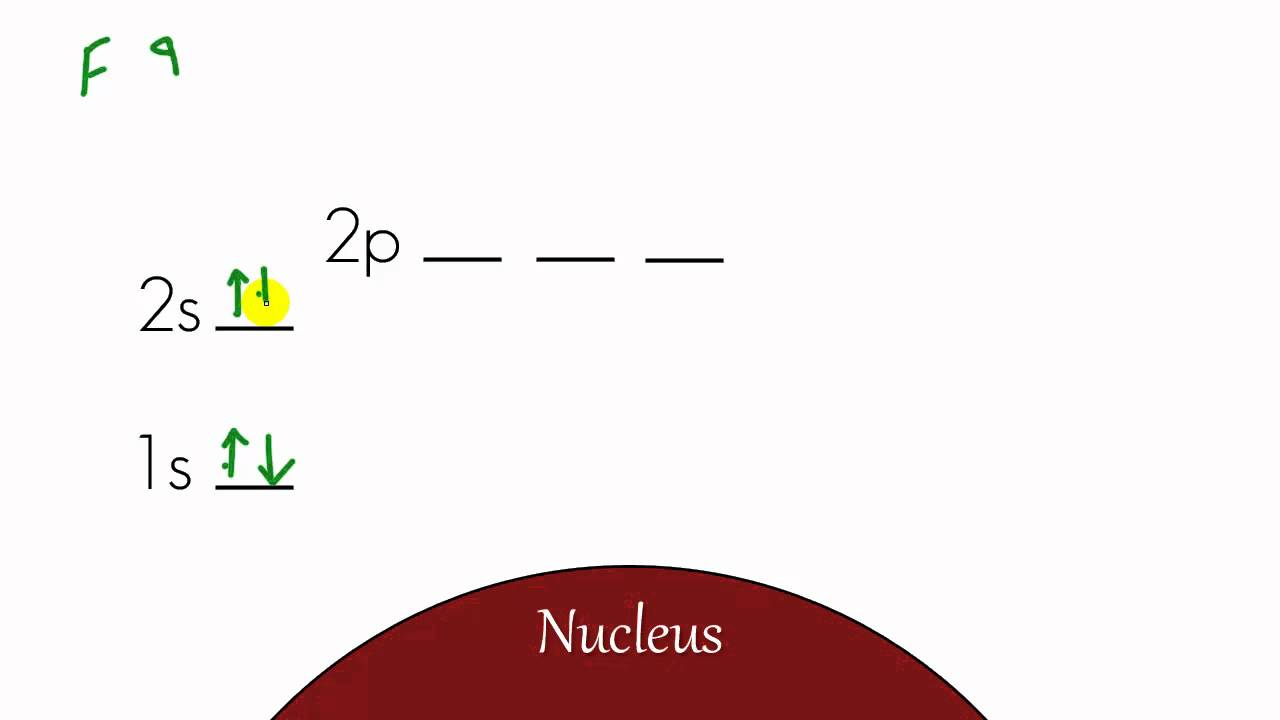

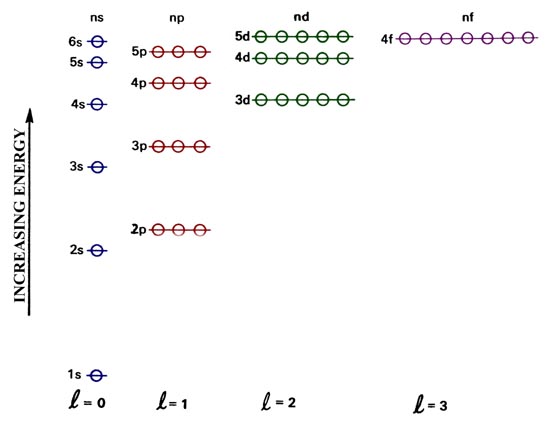













0 Response to "39 energy level diagram chemistry"
Post a Comment