40 bohr diagram for mg
MCQ Questions Answers for Chapter 2 Structure of Atom Class 11 Chemistry. Question. The triad of the nuclei that is isotonic, is. (a) 6 c 14 ' 7 N 14 ' 9 F 19. (b) 6 c 14 ' 7 N 15 ' 9 F 17. (c) 7 c 14 ' 7 N 14 ' 9 F 17. (d) 6 c 12 ' 7 N 14 ' 9 F 19. Answer. The Bohr Model of Magnesium(Mg) has a nucleus that contains 12 neutrons and 12 protons. This nucleus is surrounded by three-electron shells named K-shell, ...
17 Dec 2015 — Magnesium has 12 protons and 12 electrons. The first electron shell of a Bohr model holds 2 electrons. The second holds 8 .1 answer · duch.sd57.bc.ca Magnesium has 12 protons and 12 electrons. The first electron shell of a Bohr model holds 2 electrons. The second holds 8. So far, ...
Bohr diagram for mg
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds.Oxygen is Earth's most abundant element, and after hydrogen and helium, it is the third-most abundant element in the universe. In 0.5 M H 2 SO 4, the 1.5-μm-thick 1T-MoS 2 QS electrode delivers C a values of less than 0.2 F cm −2 over the entire scan rates because of its low 1T-MoS 2 loading (0.75 mg cm −2 ... Bohr diagram: Group No. Lewis Dots: Mg: 12: 2 - 8 - 2: 2: 2: O: 8: 2 - 6: 6: 6: Write the Lewis symbols for each atom. See graphic on the left. Determine the numbers of electrons which the atoms will lose and gain by applying the Octet Rule. Mg loses two electrons to have an octet. Oxygen gains two electrons to have an octet. Mg Atom: Mg Ion: O ...
Bohr diagram for mg. In contrast, chlorine and sodium have seven and one electrons in their.A Bohr diagram depicts an atom with a small, central nucleus and the electrons in their valence shells. The first valence shell contains 2 electrons, and the second and third shell have 8 electrons each, and the number keeps growing. The simple answer: The elements will lie in the s, p, d or f block will completely depend upon the subshell in which the last electron will enter. For example; the electron configuration of Sodium is [Ne] 3s 1 . So the last electron of Sodium enters the s-subshell or s-orbital. Hence, sodium is the s-block element. Electron beam shaping by sculpted thin films relies on electron-matter interactions and the wave nature of electrons. It can be used to study physical phenomena of special electron beams and to develop technological applications in electron microscopy that offer new and improved measurement techniques and increased resolution in different imaging modes. In this Perspective, we review recent ... Replacement eaton fuller air shift knob please verify oem number before purchase. This is the actual shift er for 18 speed eaton fuller transmission. Direct aftermarket replacement for oe a 69 18 a 5013 shift the transmission between the top two gears. Buy eaton fuller 4300764 diagram. And above and gcws over 90000lbs.
Cat 3126 engine diagram starter best wiring library cat c7 acert engine diagram water pump cat c15 fuel system caterpillar 3126 diagrams cat 3126. Installing a heui pump on a c7 or c9 cat engine 14 simple steps for a fast and proper installation. Diagram of components for the heui fuel system above 1 unit injector hydraulic pump 2 oil flow to. Where To Download Electron Dot Diagram Worksheet With Answers ... Lewis Dot Diagram Worksheet. Use the Bohr models to determine the number of valance electrons. Once you have found the number of valance electrons, place ... Identify the charges = Mg. 2+ F. 1. Cross the Charges, Mg. 2+ F. 1 = Mg. 1. F. 2. ... Lewis Dot Structures Worksheet Lewis ... Bohr diagram and Lewis Structure. ... For each of the following draw the Lewis Dot Structure give the electron arrangement EA and the molecular geometry MG. The last three slides are related to the Atomic Math Challenge that I use with the lesson. Buen Viaje 1. BSB Ch5-6 - 80 cards. BU LS111 Test 2 - 82 cards. Conclusion. The concepts of Lewis Structure, Molecular Geometry, and Hybridization hold great significance in understanding the structure, geometry, and subsequently the behavior of a substance, which is a direct result of the properties of associated element's atoms.
Atomic no. Protons, Neutrons and Electrons of all the Elements: Shell Diagram: 1: Hydrogen has 1 proton, 0 neutron and 1 electron: 2: Helium has 2 protons, 2 neutrons and 2 electrons: 3: Lithium has 3 protons, 4 neutrons and 3 electrons: 4: Beryllium has 4 protons, 5 neutrons and 4 electrons: 5: Boron has 5 protons, 6 neutrons and 5 electrons: 6: Carbon has 6 protons, 6 neutrons and 6 electrons Yamaha Warrior Wiring. 2011 yamaha warrior wiring diagram full yfm350xp atv starter relay raptor 1990 blue tra 2000 350 yfm35xm 2004 yfm35xs yb100 96 need schematic plz sportissimo html issues 2002 yfm35xp forum i have a 1996 and 208 v 90 crx wire 1989 yfm350x service manual cdi help xj550 blaster page 10 blasterforum com 250 2018 1987 starting issue ignition 87 04 carburetor honda cr for. Magnesium has 12 protons and 12 electrons. The first electron shell of a Bohr model holds two electrons. The second holds 8. So far, ...1 answer · Top answer: Hint :We know that by drawing a nucleus with required number of shells. Since the total number of electrons in a magnesium atom is $ 12. $ so start them ... Out that these four quantum numbers, however, Bohr postulated that just the major quantum number, n, determines the energy of the electron. Therefore, the 3s orbital (l=0) has actually the same energy as the 3p (l=1) and 3d (l=2) orbitals, regardless of a difference in l values. This postulate, however, holds true only for Bohr"s hydrogen atom ...
The following figure shows the probability density diagrams of Is and 2s atomic orbitals. These diagrams appear like a cloud. The electron cloud of 2s orbital shows one node, which is a region with nearly zero probability density and displays the change of sign for its corresponding wavefunction. Diagram: Question 55.
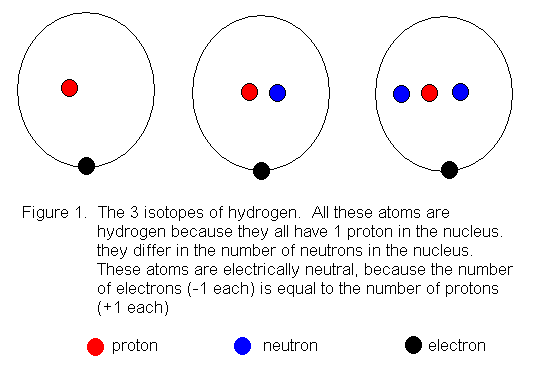
15 Aug 2020 — Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are ...
Draw a Bohr model for elements with atomic numbers 1-18 (Hydrogen thru Argon). Draw each diagram neatly, clearly labeling the correct number of protons, electrons, and neutrons for each diagram. Use the average atomic mass from the periodic table for the mass number (round to the nearest whole number).
39 private equity fund structure diagram. Private Equity Fund structure - Master-Feeder Fund A Feeder Fund is an investment vehicle that consists in the pooling of capital commitments from investors. A Feeder Fund invests, or feeds this capital, into an umbrella fund, often called a Master Fund (Main), which directs and oversees all ...
Part 1: Bohr Diagrams. 1. Complete the following table by drawing the correct Bohr models for each element. Hydrogen. Lithium. Magnesium. Oxygen. Chlorine.3 pages
Hemoglobin or haemoglobin (spelling differences) (from the Greek word αἷμα, haîma 'blood' + Latin globus 'ball, sphere' + -in) (/ ˌ h iː m ə ˈ ɡ l oʊ b ɪ n, ˈ h ɛ m oʊ ˌ-/), abbreviated Hb or Hgb, is the iron-containing oxygen-transport metalloprotein in the red blood cells (erythrocytes) of almost all vertebrates (the exception being the fish family Channichthyidae) as well ...
Symbol: Mg Atomic Number: 12. Atomic Mass: 24.305 amu ... Number of Protons/Electrons: 12. Number of Neutrons: 12 ... [Bohr Model of Magnesium]
Drawing the Bohr model for an atom gives you valuable information about the atom's valence electrons. The valence electrons— those in the outermost energy leve l-are the ones that determine the chemical properties of an atom. In order to draw a Bohr model you must first use the periodic table to tell you the number of protons, neutrons, and electrons in an atom.
The lewis dot structure for magnesium is an mg with 2 dots which stand for its two valence electrons. What is an bond electron transfer how electron dot diagram for s awesome sulfur atom flow block orbital diagram for magnesium awesome lewis electron dot diagram s. Magnesium reacts with sulfur to produce sulfide a in. That is, magnesium is a ...
Snc1p bohr diagram worksheet name: Na mg al si p s cl … Electrons in the valence shell, which is the outermost shell in an atom that contains electrons ____ compound c. Some have been done for you. • named in honour of _____, a danish physicist who developed several models for showing the arrangement of electrons in atoms. Electron 0 n 2p ...
Q#1) What is atom? Write in short words about history of atoms? Answer: In 585 B.C, Ancient Greek philosopher Democritus suggested that matter is composed of tiny indivisible particles called atoms. The name ATOM was derived from the Latin word Atomos meaning indivisible.
Drawing the Bohr model for an atom gives you valuable information about the atom's valence electrons. The valence electrons— those in the outermost energy leve l-are the ones that determine the chemical properties of an atom. In order to draw a Bohr model you must first use the periodic table to tell you the number of protons, neutrons, and electrons in an atom.
3 Li, elektron valensi = 1; 6 C, elektron valensi = 4. 12 Mg, elektron valensi = 2. Demikianlah pembahasan mengenai Struktur Atom - Pengertian Menurut Para Ahli, Teori, Komponen, Susunan dan Contoh semoga dengan adanya ulasan tersebut dapat menambah wawasan dan pengetahuan anda semua, terima kasih banyak atas kunjungannya.
It was already known that an. Compare the radial distances. Printable Blank Atom Diagram Automotive Wiring Diagram Chemistry Worksheets Bohr Model Atomic Structure Julius Plucker in 1859 found that a type of rays called. Atomic structure exercises pdf. Same Z different number of neutrons Atomic mass unit 12amu 112 mass of C Atomic mass or weight A averaged mass with respect to natural isotopes ...
Magnesium is a chemical element with the symbol mg atomic number 12 and common oxidation number 2. Bohr model for magnesium by jackie moore october 15 bohr diagram for magnesium satisfying the octet rule this is the answer to the bohr electron configuration drawing of magnesium after it has completed the octet rule in the table talk question of ...
Bohr diagram: Group No. Lewis Dots: Mg: 12: 2 - 8 - 2: 2: 2: O: 8: 2 - 6: 6: 6: Write the Lewis symbols for each atom. See graphic on the left. Determine the numbers of electrons which the atoms will lose and gain by applying the Octet Rule. Mg loses two electrons to have an octet. Oxygen gains two electrons to have an octet. Mg Atom: Mg Ion: O ...
In 0.5 M H 2 SO 4, the 1.5-μm-thick 1T-MoS 2 QS electrode delivers C a values of less than 0.2 F cm −2 over the entire scan rates because of its low 1T-MoS 2 loading (0.75 mg cm −2 ...
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds.Oxygen is Earth's most abundant element, and after hydrogen and helium, it is the third-most abundant element in the universe.


0 Response to "40 bohr diagram for mg"
Post a Comment