41 molecular orbital diagram for c2
Draw the molecular orbital (MO) electron diagram for the C2 molecular ion. Be sure your diagram contains all of the electrons in the ion, including any core electrons Energy Explanation Check ; Question: Draw the molecular orbital (MO) electron diagram for the C2 molecular ion. Be sure your diagram contains all of the electrons in the ion ... For this, we need to determine the bond order for each species. The bond order tells us the stability of a bond: a higher bond order means the bond is more stable. Step 1: Calculate the total number of valence electrons present. Step 2: Draw the molecular orbital diagram. Step 3: Calculate the bond order of the molecule/ion.
Our videos prepare you to succeed in your college classes. Let us help you simplify your studying. If you are having trouble with Chemistry, Organic, Physics, Calculus, or Statistics, we got your back! Our videos will help you understand concepts, solve your homework, and do great on your exams.
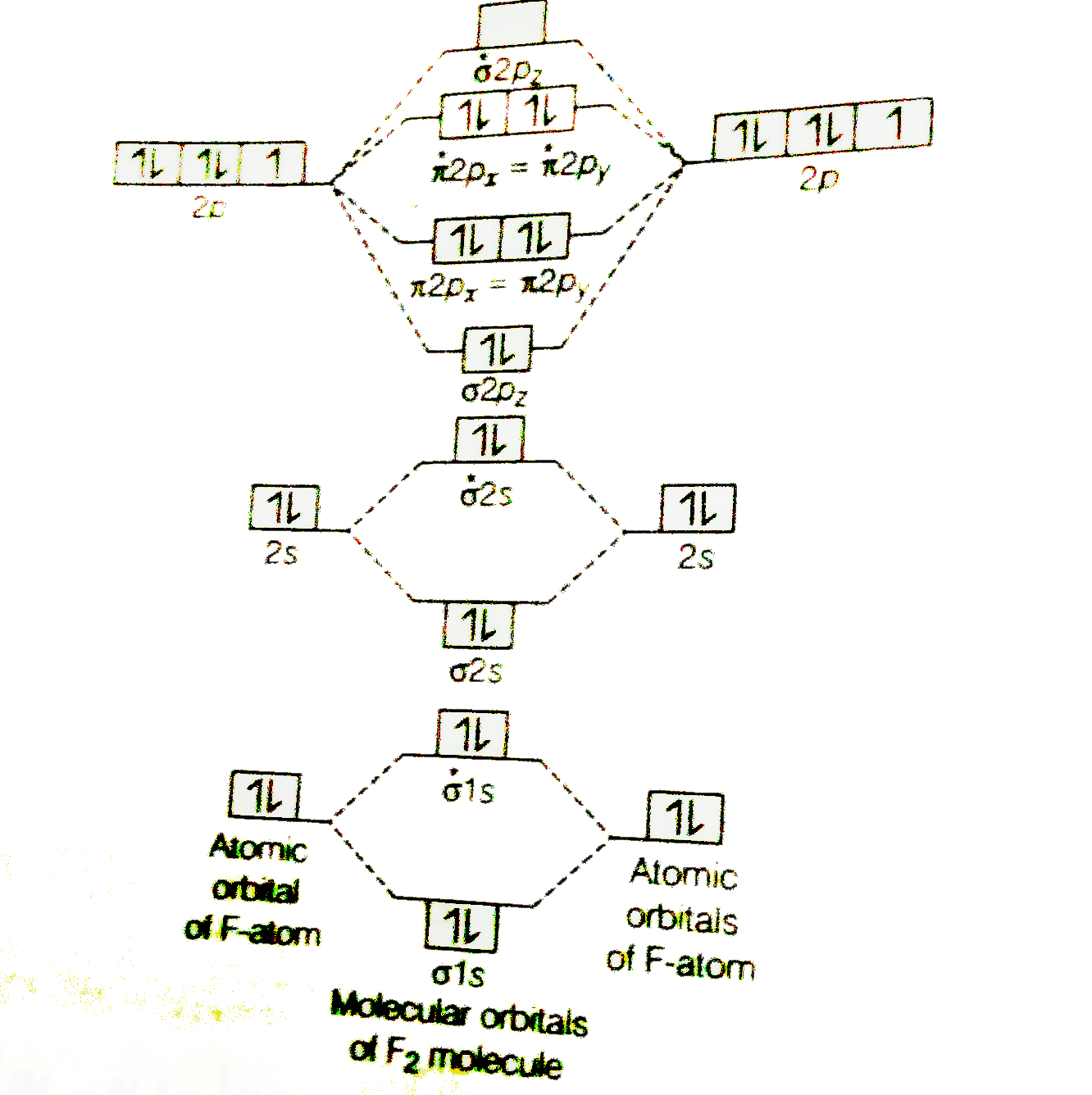
Molecular orbital diagram for c2
Both C2 and B2 have zero sigma bond. When you consider Molecular orbital theory you will find that C2 molecule has 0 sigma bond and 2 pie bond. In general pie ... Molecular orbital diagram for c2. This video shows the mo diagrams of the c2 n2 o2 and f2 molecules. Molecular orbitals are formed combining similar atomic orbitals. Just because some chemical species shows integral value of bond order doesnt mean that it should exist. Molecular orbital diagram for the molecule oxygen o2. C2 2 molecular orbital diagram. Ion predictions with mo diagrams. Interact and form molecular orbitals. As for bond orders it is 12e in bonding orbitals e in antibonding orbitals doing this normally just c2 is 128 42. Molecular orbital diagram for carbon dimer c2. Give the molecular orbital configuration for the valence electrons in cec22.
Molecular orbital diagram for c2. MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine When two carbons atoms bond, the pi(2p) bonding molecular orbitals are lower in energy than the sigma(2p) bonding orbitals.C2(2-) has a bond order of 3, so i... Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ... Answer (1 of 6): Short trick for bond order: C2- bond order is 12 * Example: This trick will work from total elctrone (8-20 )electrone.
Answer (1 of 5): C2 exists, but only above 3,642 °C (6,588 °F) i.e. in vapor state Explain why the relative energy levels diagrams for Li2, Be2, B2, C2, N2 are different The molecular orbital theory of Li2 to F2 gives a graphical explanation. Energy level diagram for Molecular orbitals. May 25, By Mrs Shilpi Nagpal 9 . It is paramagnetic in nature. 6)Li2. Molecular orbital energy level of Li2.Molecular orbitals of Li 2, Be 2 ... Molecular orbital diagram for nitrogen monoxide, the nitrosyl cation and the nitrosyl anion 1 Order of filling of molecular orbitals in heteronuclear diatomic molecules such as CO. Mar 26, · This video shows the MO diagrams of the C2, N2, O2 and F2 molecules.A molecular orbital (MO) energy level diagram - Parkway C-2Use the molecular orbital ... From the periodic table as we have already discussed the Molecular orbital diagrams of diatomic molecules of 1st two periods starting from ...
a. Determine the bond order for each molecule b. Determine whether each of the molecules is paramagnetic or diamagnetic. c. Determine which molecule has the ... Bonding in Heteronuclear Diatomics Molecular orbital diagrams for heteronuclear diatomics (AB) are constructed in a similar manner for homonuclear diatomics, but there is an important difference resulting from the fact that the energies of the atomic orbitals of A and B are different. Specifically, the MO diagram is skewed, with the atomic orbitals of the more electronegative element (A) being ... This diagram should be to scale. If necessary, you can omit the two lowest molecular orbitals from the diagram. Write the molecular orbital configuration for your molecule. Calculate the bond order for your molecule. Jen B2 Jeff OH- Mike F C2 Laura Li2 Jill Be2 Cody N2 Diana CO Kevin BF Wai BH Mike S NH Ly HF Jake SH Melissa F2 10.8K answers. 116M people helped. Bond order = 1/2 (number of electrons in bonding orbitals - number of electrons in antibonding orbitals) Therefore, Bond order of C2+ = 1/2 (5 - 2) = 3/2 = 1.5. Bond order of C2- = 1/2 (7 - 2) = 5/2 = 2.5. Bond order of C2 = 1/2 (6 - 2) = 2. Highest bond order means highest bond energy and shortest bond length ...
C2 molecular orbital diagram. A diatomic molecular orbital diagram is used to understand the bonding of a diatomic molecule. Get 11 help now from expert chemistry tutors. Fill from the bottom up with 8 electrons total. They also give insight to the bond order of the molecule how many bonds are shared between the two atoms.
Answer. Use molecular orbital theory to describe the bonding in the following. For each one, find the bond order and decide whether it is ...
The molecular orbital diagram for c22 this problem has been solved. C2 molecular orbital diagram. As you can see in the diagram the two 2ppi orbitals lets say 2ppix and 2ppiy are the highest energy occupied molecular orbitals. Mo diagrams can be used to deduce magnetic properties of a molecule and how they change with ionization.
4 Lecture 2 Pi bond (π): bonding molecular orbital -The bonding electron density lies above and below, or in front and in back of the bonding axis, with no electron directly on the bonding axis, since 2p orbitals do not have any electron density at the nucleus.
Molecular Orbital Diagram - Cl2, Br2, I2 3s & 3p and higher atomic orbitals are not so widely separated in energy and allow significant mixing (hybridization) to occur. This mixing causes the inversion of the σσand πmolecular orbitals' energy. σσσ ππ σ* π* 3,4,5 p 3, 4,5 s σ* σ 3,4,5 s 3,4,5 p Interhalogens Br Br F F Br F F F F.
The molecular orbital diagram for C 2 molecule is :. The electronic configuration of C 2 is K K (σ2s) 2 (σ * 2s) 2 n(2px) 2 n(2py) 2. The C 2 molecule is diamagnetic because all electrons are paired there are no unpaired electrons.
Molecular orbital theory shows that it has two sets of paired electrons in a degenerate bonding set of orbitals. This gives a bond order of two, ...
Match. Gravity. Item 1: Part A. By drawing molecular orbital diagrams for B2, C2, N2, O2, and F2, predict which of these homonuclear diatomic molecules are magnetic. By drawing molecular orbital diagrams for , , , , and , predict which of these homonuclear diatomic molecules are magnetic. F2.
Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons Draw the molecular orbital diagram for the oxygen molecule, O 2. From this diagram, calculate the bond order for O 2. How does this diagram account for the paramagnetism of O 2? Solution. We draw a molecular orbital energy diagram similar to that shown in Figure 11.
Molecular orbital theory shows that it has two sets of paired electrons in a degenerate $\pi $- bonding set of orbitals. This gives a bond order of 2, which ...
Molecular Orbital Diagram for Carbon Dimer (C2).Fill from the bottom up, with 8 electrons total.Bonding Order is 2, ...
Draw the molecular orbital diagram for C2. The number of electrons in the σ2p molecular orbital is ____. 4. 137.) Draw the molecular orbital diagram for N2. The number of electrons in antibonding molecular orbitals is ____. 2. 138.) Draw the molecular orbital diagram for N2. The number of electrons in the σ2p molecular orbital is ____.
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ...
The lowest energy unoccupied molecular orbital is 2pσ, so that is where the extra electron will be added. The electron configuration of the neutral C2 molecule is -- I'll use the notation given to you in the diagram. C2:(1sσ)2(1s* σ)2(2sσ)2(2s* σ)2(2pπ)4. The electron configuration of the C− 2 ion will be.
C2 n2 o2 and f2 molecules diego troya. Molecular orbital diagram for carbon dimer c2. Molecular orbital diagram for the molecule oxygen o2. In the mo approach each carbon atom has four valence orbitals namely a 2s and three 2p. At the left of the table the energies are very close to each other and as a result the 2s and 2p orbitals mix with ...
C2 molecular orbital diagram. A mo is defined as the combination of atomic orbitals. As for bond orders it is 12e in bonding orbitals e in antibonding orbitals. The only orbitals that are important in our discussion of molecular orbitals are those formed when valence shell orbitals are combined.
Energy level diagram for Molecular orbitals. ... If N b = Na,the molecule is again unstable because influence of electrons in the antibonding molecular orbital is greater than the bond influence of electron in the bonding molecular orbitals. 2) Stability of molecules in terms of bond order.
Molecular Orbital C2- Diagram, Bond Order, Magnetism. Postby Brooke Tobias 1B » Fri Nov 27, 2015 7:31 pm. A video explaining the molecular orbital diagram and notation of C2- along with the bond order and magnetism. You do not have the required permissions to view the files attached to this post. Top.
C2 2 molecular orbital diagram. Ion predictions with mo diagrams. Interact and form molecular orbitals. As for bond orders it is 12e in bonding orbitals e in antibonding orbitals doing this normally just c2 is 128 42. Molecular orbital diagram for carbon dimer c2. Give the molecular orbital configuration for the valence electrons in cec22.
Molecular orbital diagram for c2. This video shows the mo diagrams of the c2 n2 o2 and f2 molecules. Molecular orbitals are formed combining similar atomic orbitals. Just because some chemical species shows integral value of bond order doesnt mean that it should exist. Molecular orbital diagram for the molecule oxygen o2.
Both C2 and B2 have zero sigma bond. When you consider Molecular orbital theory you will find that C2 molecule has 0 sigma bond and 2 pie bond. In general pie ...








0 Response to "41 molecular orbital diagram for c2"
Post a Comment