42 silicon electron dot diagram
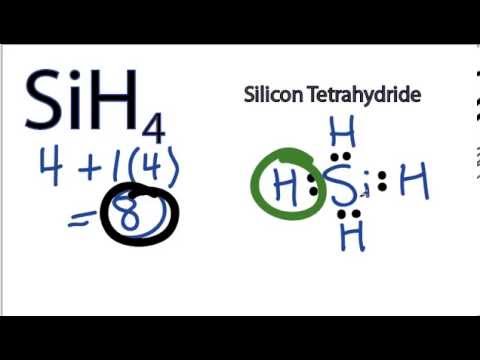
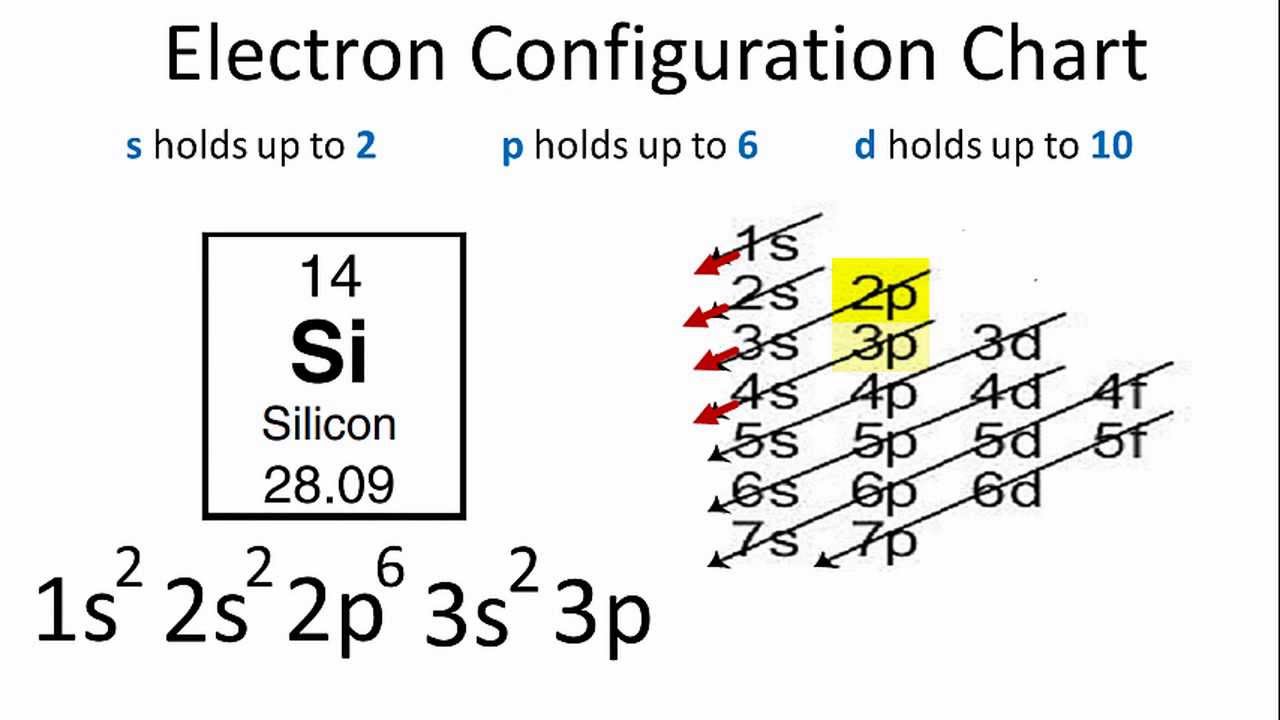
1:38A step-by-step explanation of how to draw the SiH4 Lewis Dot Structure (Silicon Tetrafluoride).For the SiH4 ...11 May 2013 · Uploaded by Wayne Breslyn Electron Dot Diagram for Silicon. what is the electron dot diagram for silicon answers the formula for silicon oxide is sio 2 to do the electron dot diagram first you put the symbol for the metal silicon in the middle and then you put electron configuration for silicon si how to write the electron configuration for silicon si in order to write the silicon electron configuration we first need ...
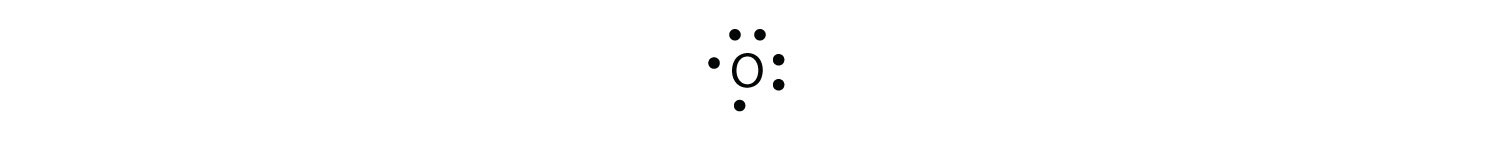
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ...
Silicon electron dot diagram
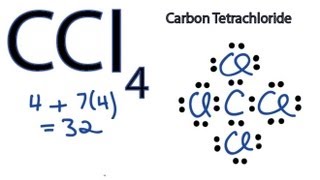
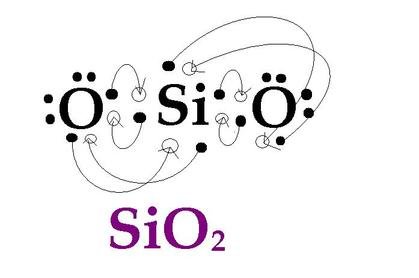
Best Answer. Copy. There are four valance electrons in Silicon, therefore there will be 4 dots in your electron dot diagram. Wiki User. ∙ 2011-03-02 02:37:57. This answer is: Helpful. Not ... The electron and molecular geometry of SiO2 are linear. The bond angle of Silicon dioxide is 180º and the hybridization of it is Sp. The total valence electron available for the Silicon dioxide lewis structure is 16. The formal charge in the SiO2 lewis dot structure is zero. SiO2 is a non-polar molecule. The dot structure for silicon tetraflouride starts with the Si atom in the middle. On each cardinal side is a singly bonded F atom, each with six dots.
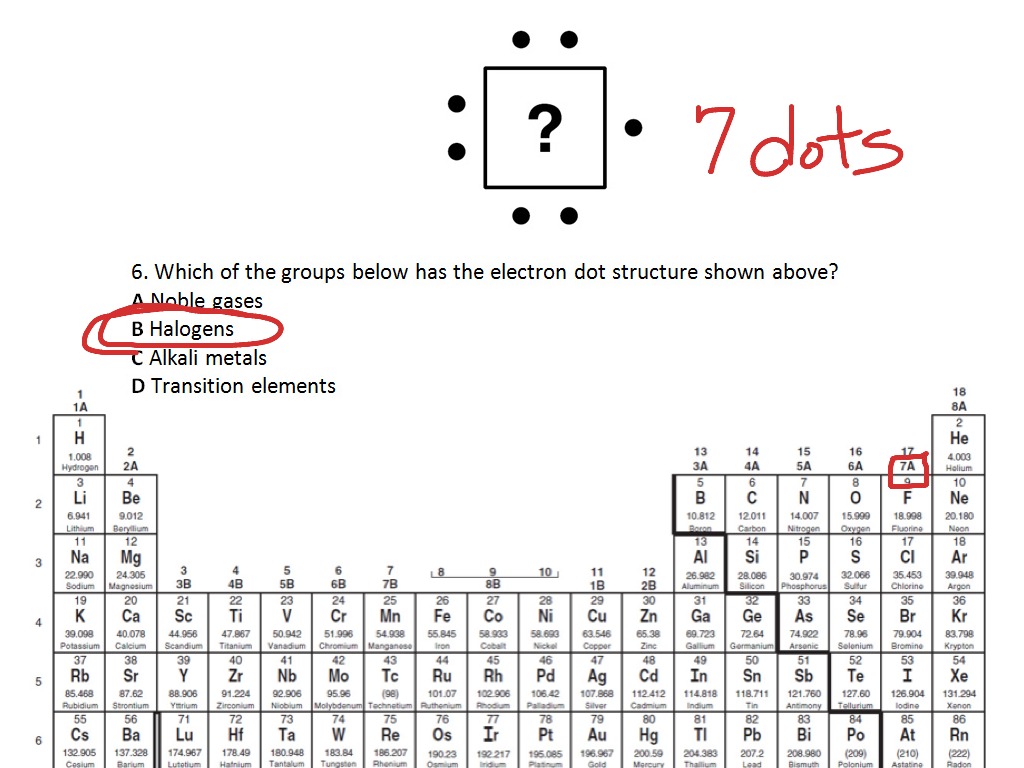
Silicon electron dot diagram. 1:00A step-by-step explanation of how to draw the SiH4 Lewis Dot Structure (Silicon Tetrahydride).For the SiH4 ...26 May 2013 · Uploaded by Wayne Breslyn Lewis dot diagram for silicon. The key is to understand the steps and practice. Since it is in group 4 it will have 4 valence electrons. Sublimes with decomposition at 2700c. The letter c is the kernel. The lewis dot diagram for neon has a pair of electrons on each side of neon symbol ne for a total of 8 electrons. Silicon tetrachloride sif. In SiO2 Lewis Dot Structure, the Silicon atom is a bigger atom and attaches with four oxygen atoms to form a single bond at tetrahedral angles. Silicon Dot Diagram. what is the electron dot diagram for silicon answers argon has eight valence electrons in a dot diagram there are eight dots located around an element in the cardinal directions two dots to be in place of what is the lewis dot structure for silicon how is it silicon is in group 14 sometimes called group iv or 4 since it is in group 4 it will have 4 valence electrons when ...
1:24A step-by-step explanation of how to draw the SiCl4 Lewis Dot Structure (Silicon tetrachloride).For the SiCl4 ...22 May 2013 · Uploaded by Wayne Breslyn A step-by-step explanation of how to draw the Lewis dot structure for Si (Silicon). I show you where Silicon is on the periodic table and how to determine h... Lewis Dot Diagrams of Selected Elements. Lewis Symbols: Electron Configuration into Shells: Index Chemical concepts Chemistry of the Elements Periodic Table . HyperPhysics***** Quantum Physics : R Nave: Go Back: Electron Distributions Into Shells for the First Three Periods. A chemical element is identified by the number of protons in its ... Silicon is in Group 14 (sometimes called Group IV or 4). Since it is in Group 4 it will have 4 valence electrons. When you draw the Lewis structure for Silicon ...2 answers · 1 vote: Apparently there are different schools of thought on this (How to Draw Electron Dot Diagrams ...
Silicon is particularly interesting to scientists because it shares so many similar traits with carbon. Both elements are found in group 14, which means they ... Electron dot structure of silicon 1 See answer Advertisement Advertisement n45 is waiting for your help. Add your answer and earn points. nikhita nikhita Silicon is in Group 14 ). Since it is in Group 4 it will have 4 valence electrons. When you draw the Lewis structure for Silicon you'll put four "dots" or valance electrons around the element ... The dot structure for silicon tetraflouride starts with the Si atom in the middle. On each cardinal side is a singly bonded F atom, each with six dots. The electron and molecular geometry of SiO2 are linear. The bond angle of Silicon dioxide is 180º and the hybridization of it is Sp. The total valence electron available for the Silicon dioxide lewis structure is 16. The formal charge in the SiO2 lewis dot structure is zero. SiO2 is a non-polar molecule.
Best Answer. Copy. There are four valance electrons in Silicon, therefore there will be 4 dots in your electron dot diagram. Wiki User. ∙ 2011-03-02 02:37:57. This answer is: Helpful. Not ...

Si Silicon Element Information Facts Properties Trends Uses And Comparison Periodic Table Of The Elements Schoolmykids




















0 Response to "42 silicon electron dot diagram"
Post a Comment