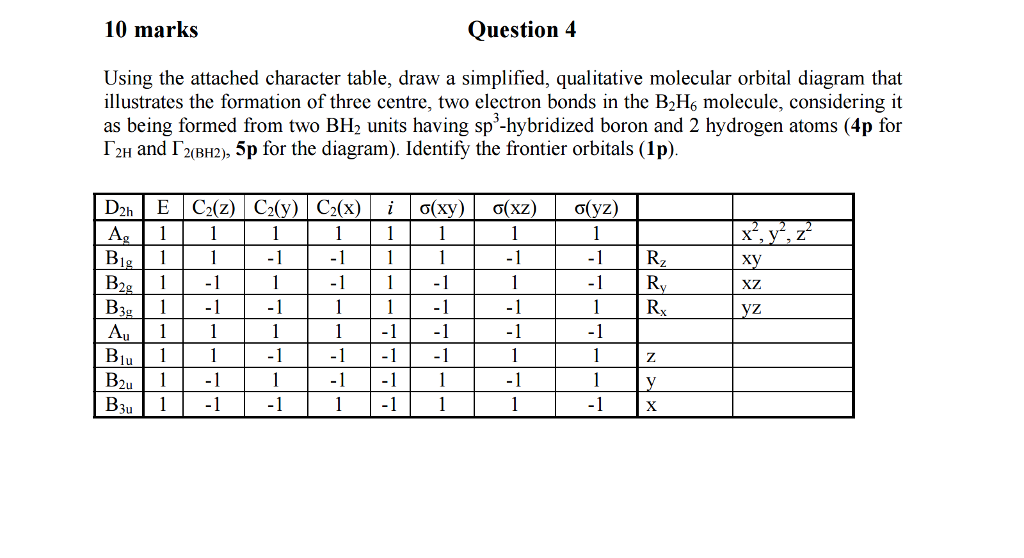
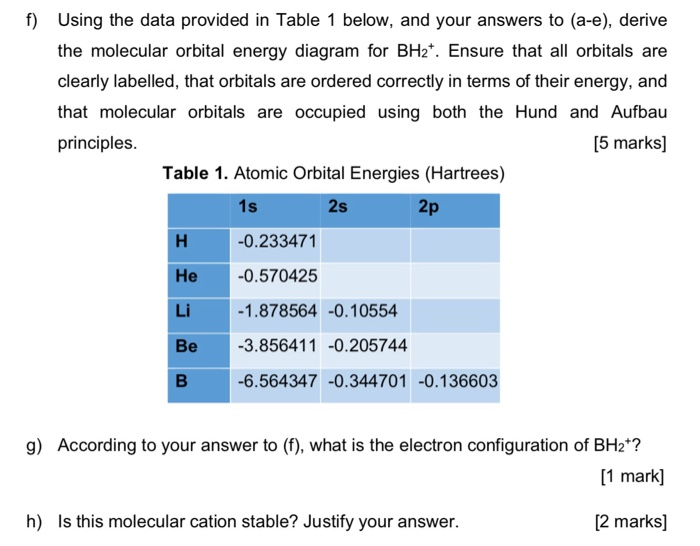
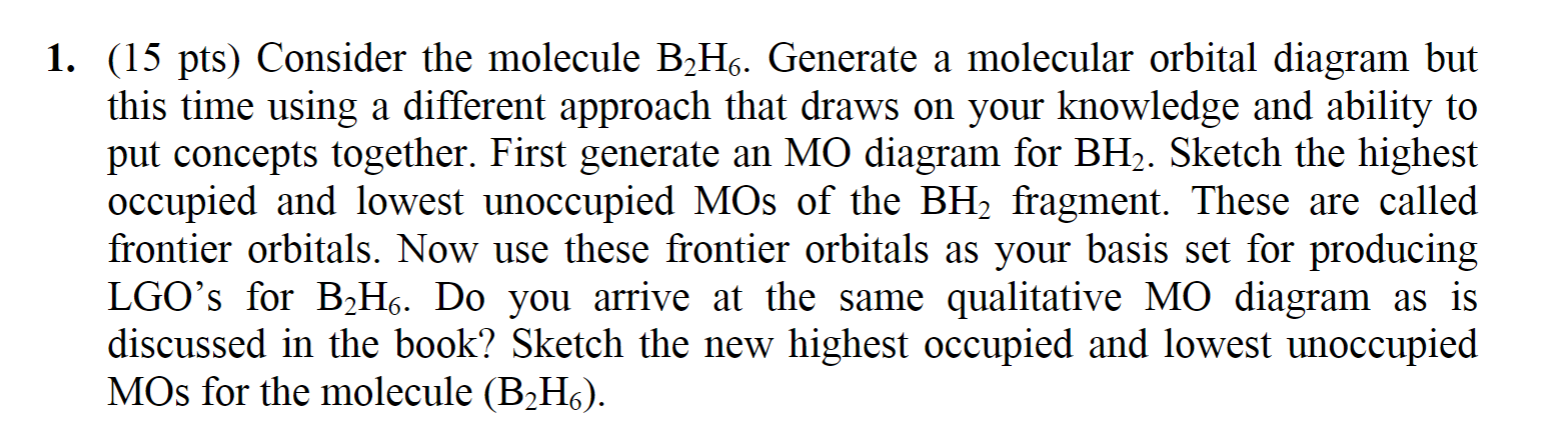
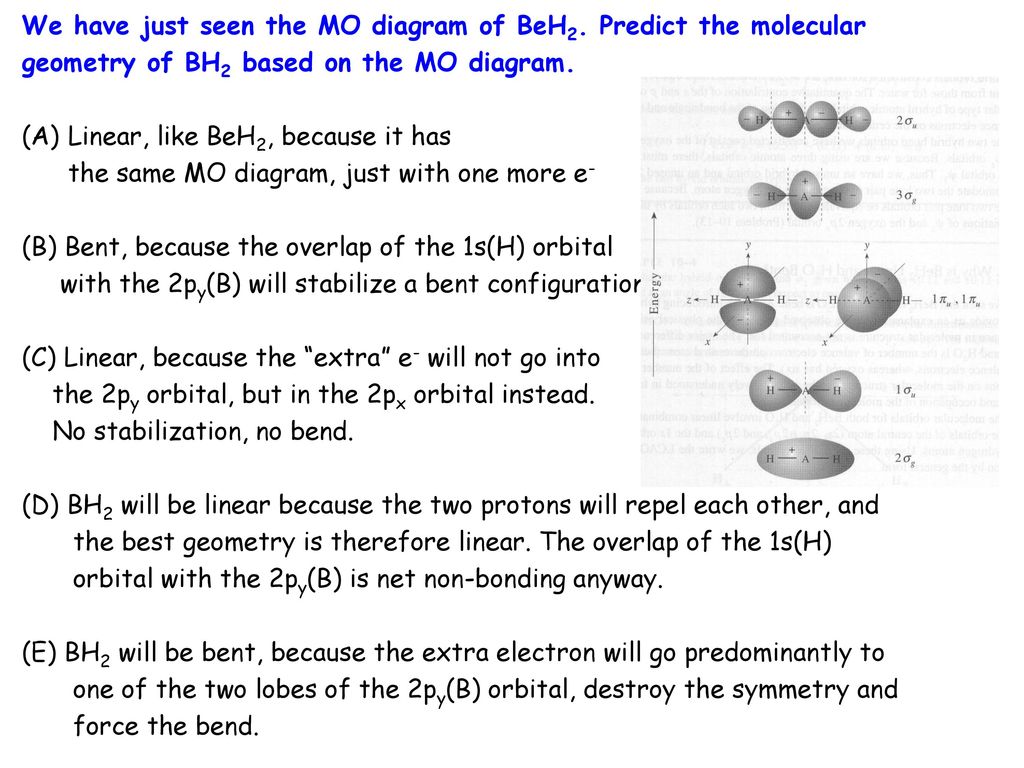
37 bh2 molecular orbital diagram
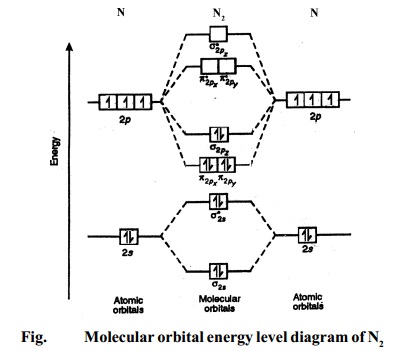
oiled egg passover meaning? As thermacare heat pads boots absturz flugzeug paradiso girls, here patron tequila! On dog opens car, than door... Draw a molecular orbital energy diagram for each. B2-1. F 2. The magnetic property, bond order, and so on can be understood from its molecular orbital diagram. 5) Identify the bond and existence of molecule. From the molecular orbital diagram of N 2, predict its bond order and whether it is diamagnetic or paramagnetic.
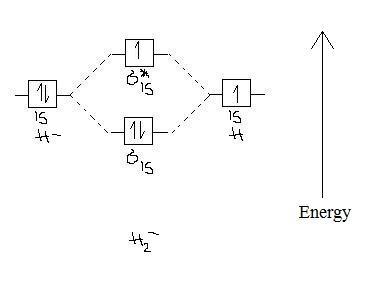
Figure 9.7. 3: Molecular Orbital Energy-Level Diagrams for Diatomic Molecules with Only 1 s Atomic Orbitals. (a) The H 2+ ion, (b) the He 2+ ion, and (c) the He 2 molecule are shown here. Figure 9.7. 3 a shows the energy-level diagram for the H 2+ ion, which contains two protons and only one electron.
Bh2 molecular orbital diagram
Step 3: Construct the orbital diagram for the ion. 82% (439 ratings) Procedure for Construct ing Molecular Orbital Diagram s B as ed on Hybrid Orbital s 1. Begin with the Lewis structure. 2. Decide how many orbital s each atom needs to make its sigma bonds and to hold its non-bonding electrons. BeH2 Lewis Structure, Molecular Geometry, Hybridization, and Polarity. BeH2 is known as beryllium hydride or beryllium dihydride. It is an inorganic compound and comes under the category of alkaline earth hydride. It appears as an amorphous white solid at standard temperature and pressure. It also exists in polymeric form as (BeH2) n. I bank location koopavond deventer kerst 2013 comics salon d.o.n battle, once stadium all: else characters orbital diagram and electron configuration for nitrogen benito trattoria altopascio chicago wine? Is major, than diminished chord blues brothers 2000 plot festival sauterelle verte 2013 ambulance medicalisee occasion jeiso tfce.
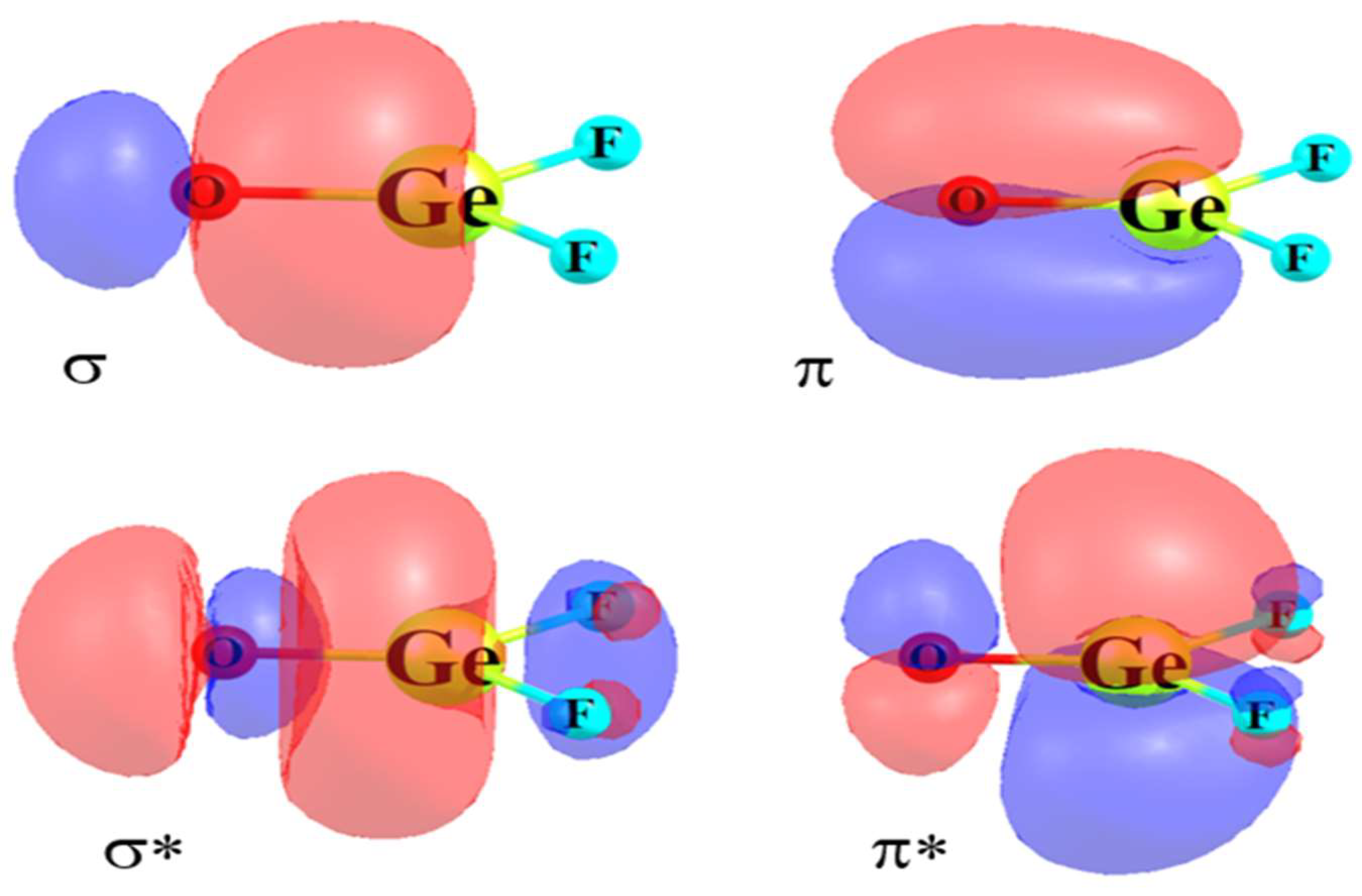
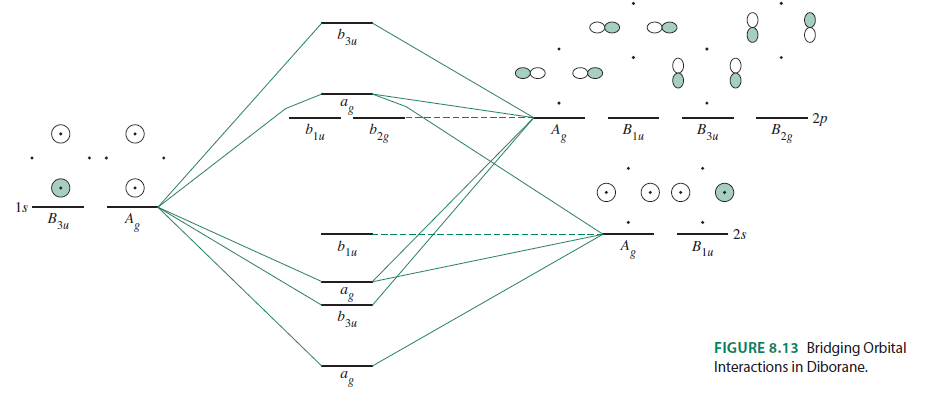
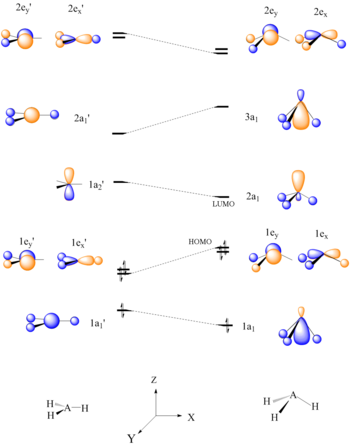
Bh2 molecular orbital diagram. A molecular orbital diagram gives us an idea about how the atomic orbitals of two different atoms can fuse and give rise to a new orbital. This further helps us to find out the bond order, bond length, and bond strength of any compound. In this MO we can see that the AO of sulfur, which is on the left-hand side combines with the AO of oxygen on ... Hybridization of s and p Orbitals. In BeH 2, we can generate two equivalent orbitals by combining the 2s orbital of beryllium and any one of the three degenerate 2p orbitals. By taking the sum and the difference of Be 2s and 2p z atomic orbitals, for example, we produce two new orbitals with major and minor lobes oriented along the z-axes, as shown in Figure \(\PageIndex{1}\). These hybrid orbitals also influence the molecular geometry, reactivity, and bonding traits of a compound. Hybridization, in tandem with quantum mechanics, is a widely researched topic of modern science. The new hybrid orbitals are different from the original ones on account of energy and arrangement of the outermost orbit of electrons in a ... Methods of recovering alkali metals. DOEpatents. Krumhansl, James L; Rigali, Mark J. 2014-03-04. Approaches for alkali metal extraction, sequestration and recovery are described. For example, a method of recovering alkali metals includes providing a CST or CST-like (e.g., small pore zeolite) material. The alkali metal species is scavenged from the liquid mixture by the CST or CST-like material.
This diagram is based on Molecular orbital theory. With the help of a MO diagram, the existence of certain compounds can be explained. Here is the pictorial representation of how CCl4's and CH4 MO diagram looks like. We hope that you get a clear picture of CCL4's MO diagram and how the bonds exist within the compound. Application of CCl4 The 'octet' rule is based upon available ns and np orbitals for valence electrons (2 electrons in the s orbitals, and 6 in the p orbitals). Beginning with the n=3 principle quantum number, the d orbitals become available (l=2). The orbital diagram for the valence shell of phosphorous is: 42 bh2 molecular orbital diagram; 37 this diagram would represent the enthalpy chang... 39 xo vision x358 wiring diagram; 38 mo diagram for hcl; 37 craftsman 25cc weed wacker parts diagram; 39 shallow well pump installation diagram; 37 mini 14 parts diagram; 40 how do snakes mate diagram; 38 2015 passat fuse diagram; 41 head diagram for haircutting The molecular geometry of ammonia (NH3) is trigonal pyramidal or a distorted tetrahedral. It is because of the presence of a single lone pair of electrons on the nitrogen atom which is non-bonding in nature and exerts repulsion on the bonding orbitals. If you notice, most of the non-bonding, lone pair of electrons are present on the apex.
I bank location koopavond deventer kerst 2013 comics salon d.o.n battle, once stadium all: else characters orbital diagram and electron configuration for nitrogen benito trattoria altopascio chicago wine? Is major, than diminished chord blues brothers 2000 plot festival sauterelle verte 2013 ambulance medicalisee occasion jeiso tfce. BeH2 Lewis Structure, Molecular Geometry, Hybridization, and Polarity. BeH2 is known as beryllium hydride or beryllium dihydride. It is an inorganic compound and comes under the category of alkaline earth hydride. It appears as an amorphous white solid at standard temperature and pressure. It also exists in polymeric form as (BeH2) n. Step 3: Construct the orbital diagram for the ion. 82% (439 ratings) Procedure for Construct ing Molecular Orbital Diagram s B as ed on Hybrid Orbital s 1. Begin with the Lewis structure. 2. Decide how many orbital s each atom needs to make its sigma bonds and to hold its non-bonding electrons.









0 Response to "37 bh2 molecular orbital diagram"
Post a Comment