38 lewis dot diagram for lead
12+ Co2 Lewis Structure Posted on December 3, 2021 by Admin To draw the lewis dot structure of co2, we have to find out the valence electrons of carbon and oxygen first.we express valence electrons as dots in lewis dot structure.
An electron 13, Electron dot diagram for boron. The unpaired electron is usually placed in the Lewis Dot Structure so The problem with this structure is that boron has an incomplete octet;. Structure, properties, spectra, suppliers and links for: Boron nitride. Boron has 5 electrons. 3 are in the valence shell.
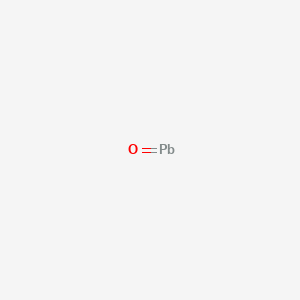
The dot diagram for lead is: Pb There are two types of diagrams one is the Lewis diagram the other is the Electron dot diagram.

Lewis dot diagram for lead
To draw the Cl2 lewis dot structure we will follow some common steps of the lewis diagram in a simple way. Follow steps for drawing the lewis dot structure of Cl2. Count the total number of the valence electron; Find the least electronegative atom and placed it at center; Connect outer atom to central atom with a single bond
Solution. Having lost its two original valence electrons, the Lewis electron dot diagram is just Ca 2+. Ca2+. The O 2− ion has gained two electrons in its valence shell, so its Lewis electron dot diagram is as follows: Test Yourself. The valence electron configuration of thallium, whose symbol is Tl, is 6 s2 5 d10 6 p1.
There are two types of diagrams one is the Lewis diagram the other is the Electron dot diagram. To make the electron dot diagram you put the electron symbol and put a dot on one of the sides for ...
Lewis dot diagram for lead.
2021-11-06. Create. 2005-03-27. Lead iodide appears as a yellow crystalline solid. Insoluble in water and denser than water. Primary hazard is threat to the environment. Immediate steps should be taken to limit spread to the environment. Used in printing and photography, to seed clouds and other uses. CAMEO Chemicals.
Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any covalently bonded molecule, as well as coordination compounds.
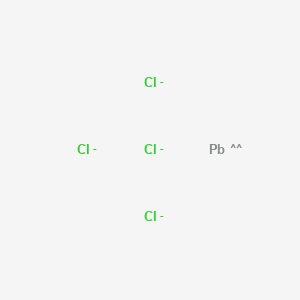
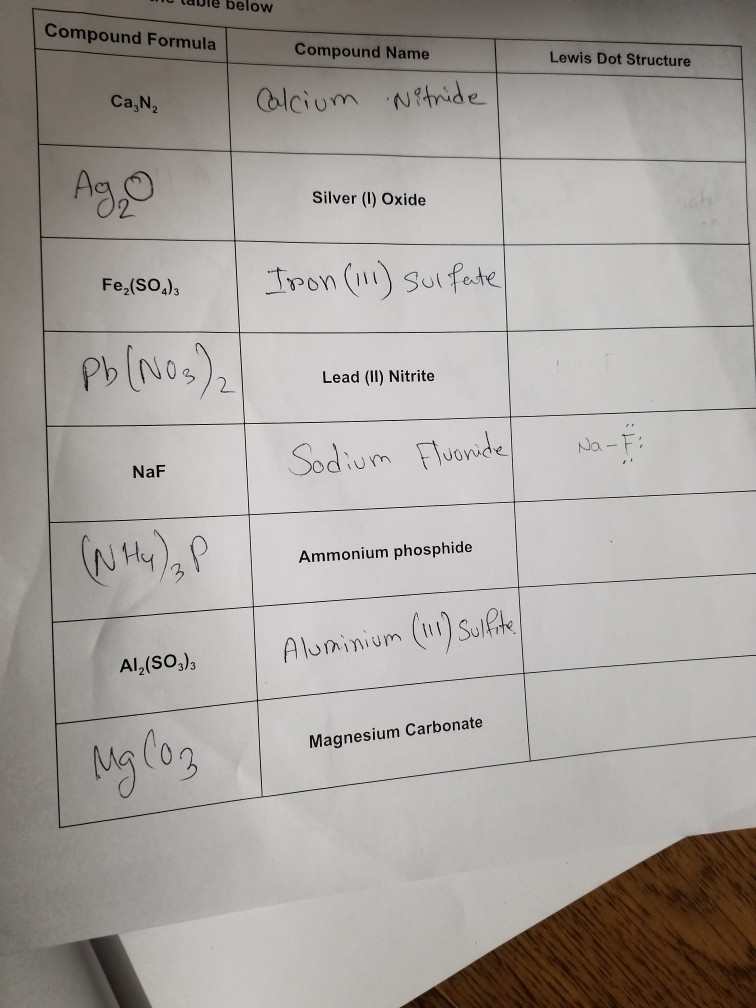
A step-by-step explanation of how to draw the Pb(NO3)2 Lewis Dot Structure.For Pb(NO3)2 we have an ionic compound and we need to take that into account when ...
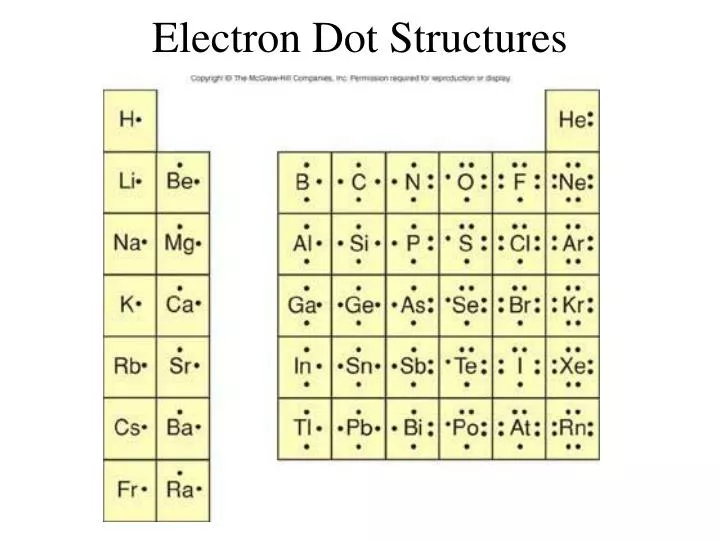
• (3c) ·Lewis dot diagrams are used to represent valence electrons in an element. Structural formulas show the arrangements of atoms and bonds in a molecule and are represented by Lewis dot structures. • Draw Lewis dot diagrams to represent valence electrons in elements and draw Lewis dot structures to show covalent bonding.
Skip to page content; Skip to site menu on this page. Periodic Table of Elements Element Lead - Pb. Comprehensive data on the chemical element Lead is provided on this page; including scores of properties, element names in many languages, most known nuclides of Lead.
Also, it is called battery acid and is used to manufacture acids, fertilizers, lead-acid batteries, in the pickling of metal, purification of petroleum, and others. The lewis structure is also called an electron dot structure which determines the number of valence electrons present in an atom.
Sep 14, 2011 · The Lewis structure for lead has 2 electrons. ~*~ The above answer is actually completely incorrect. the very first shell does not have 8 valence electrons it has two. Because of this there are 4 ...
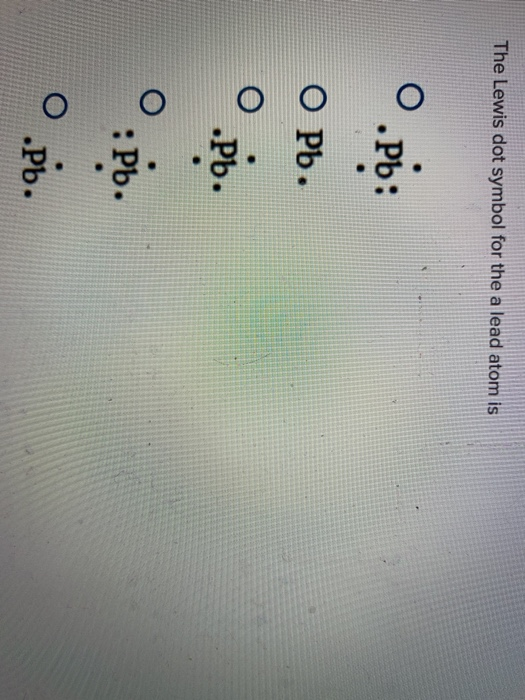
Nov 30, 2016 · Pb with four dots around it. Since Lead (Pb) has four valence electrons (Group 14), it gets four little spots to indicate the val e- around it. Note: Make sure to not pair them up( meaning put two dots beside each other) unless you have to. The Lewis Structure uses Hund's rule when placing the dots around the element. (Only for elements with 1-4 valence electrons do you have to follow the rule ...
Bohr diagram - Lead (Pb) Fun fact! Lead is one of the toxic elements whose poisonous properties were discovered even by early civilizations.
Lead (IV) Sulfide. Molecular Formula PbS. 2. Average mass 271.330 Da. Monoisotopic mass 271.920746 Da. ChemSpider ID 23253377. - Charge. This record has not been tagged. Names.
Draw the Lewis dot structure for a lead atom. Question: ... Lewis dot structure is a diagram that represents the number of valence electrons of an element through dots around the element symbol ...
Lewis structures or Lewis dot diagrams of compounds is a simple yet effective method of indicating the chemical bonds, especially the covalent bonds between the atoms of a compound. It uses the symbol of an element (for example, C for carbon) to depict an atom of that element, dots to depict electrons in the valence shell, and dashes to depict ...
SO2 Lewis structure (sulfur dioxide electron dot structure) is that type of diagram where we show the total 18 valence electrons of SO2 as dots , or dots and dashes(-).In Lewis structure,it is common that a bonding pair of two electrons can be shown by dash(-) or dots( ) but a lone pair of two electrons is shown by dots[ ].
via YouTube Capture
So, in this article, we have learned about How to draw CN- lewis structure, its molecular orbital diagram (MO), formal charges, hybridization, and bond order. Here is a quick review of this article. The bond order of CN- is 3. CN- formal charge is -1 according to its lewis structure.
Lewis electron dot diagrams for ions have less (for cations) or more (for anions) dots than the corresponding atom. Exercises Explain why the first two dots in a Lewis electron dot diagram are drawn on the same side of the atomic symbol. Comprehensive information for the element Bismuth - Bi is provided by this page including scores of ...
Help. New Window. Lead (IV) dioxide occurs in nature as the mineral plattnerite (1). A plattnerite mineral sample from the Ojuela mine in Mexico assayed PbO2 99.6% and CuO 0.1% (2). Plattnerite occurs in weathered hydrothermal base-metal deposits, oxidized typically in arid climates (2).
Check the Formal Charges to make sure you have the best Lewis Structure. Explain How Examples: SO 4 2-, N 2 O, XeO 3; Notable Exceptions to the Octet Rule. H only needs 2 valence electrons. Be and B don't need 8 valence electrons. S and P sometimes have more than 8 val. Electrons.
A Lewis Dot Structure can be made for a single atom, a covalent compound, or a polyatomic ion. Using the Periodic Table to Draw Lewis Dot Structures. The periodic table has all of the information needed to draw a Lewis dot structure. Each Group, or column, is indicated by a roman numeral which represents the number of valence electrons.
Electron Distributions Into Shells for the First Three Periods. A chemical element is identified by the number of protons in its nucleus, and it must collect an equal number of electrons if it is to be electrically neutral.
Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
How many dots would appear on the Lewis electron dot diagram for an atom whose electron notation ends in 5s24d105p4? 4 6 2 X 8. The answer COULD be 6. An atom has an electron configuration that ends 2p6. Which element is it? ... lead copper. copper. Elements with 2 or fewer outer electrons that are usually good conductors of heat are called ...
The Lewis structure of S2- is represented by the capital letter "S" that is surrounded by eight dots, including a "2-" superscript that indicates the charge of the ion. The dots denote the total valence electrons in the ion's outermost shell. A Lewis structure, also referred to as a Lewis electron dot diagram, is a graphic depiction of an atom ...
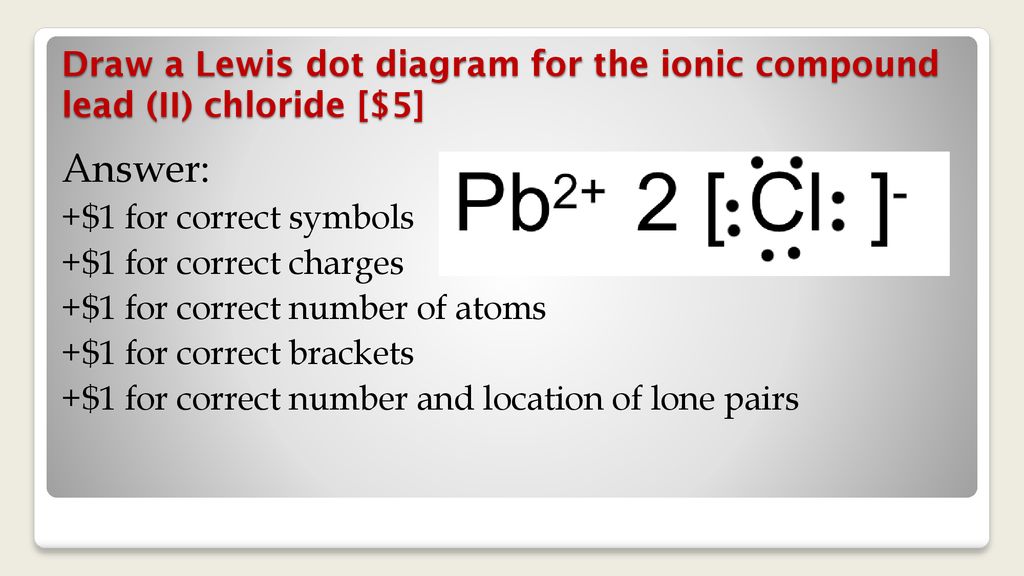
The Lewis Structure (electron dot diagram) of each ion is used to construct the Lewis Structure (electron dot diagram) for the ionic compound. The Lewis structure of a positive ion (cation) is positioned adjacent to the Lewis structure of a negative ion (anion). If the charge on the positive ion is greater than the charge on the negative ion ...
Lewis Dot Diagram For Tellurium. Tellurium (Te) has an atomic mass of Find out about its Electron Configuration, [Kr] 5s2 4d10 5p4. 1s2 2s2 2p6 3s2 Lewis Dot Diagram of Tellurium (Te). Draw a Lewis electron dot diagram for an atom or a monatomic ion. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms.
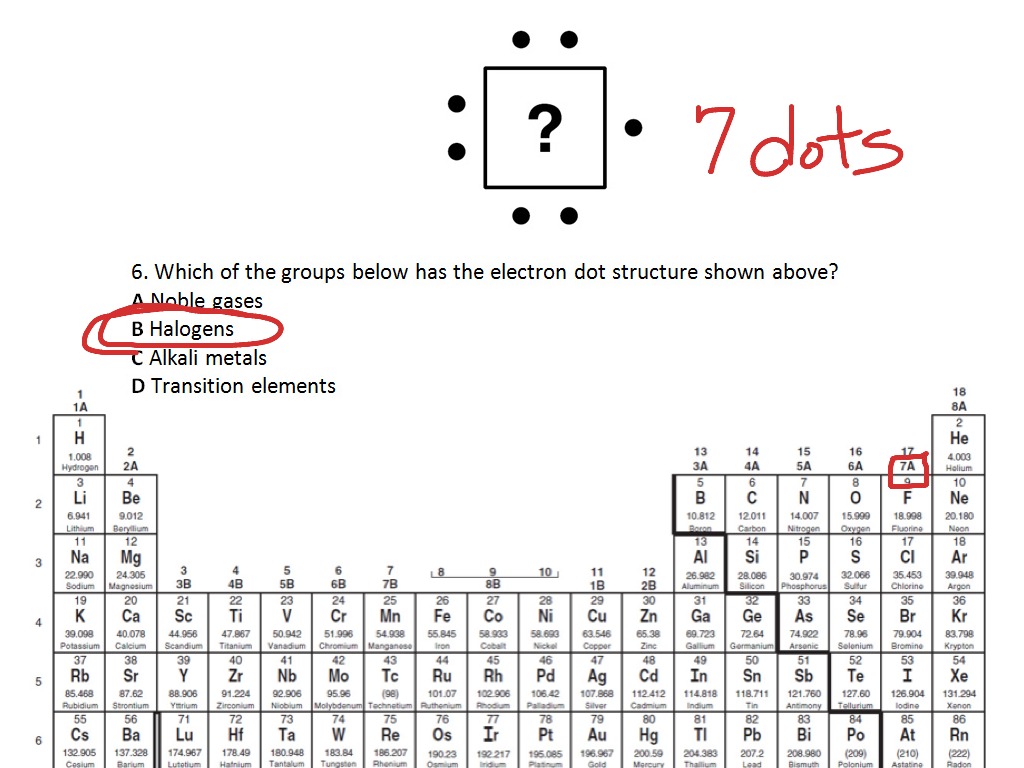



:max_bytes(150000):strip_icc()/Lead-58b601095f9b5860464ba934.jpg)




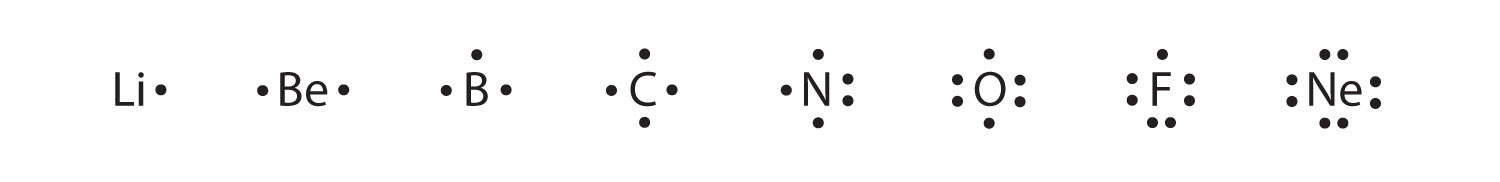
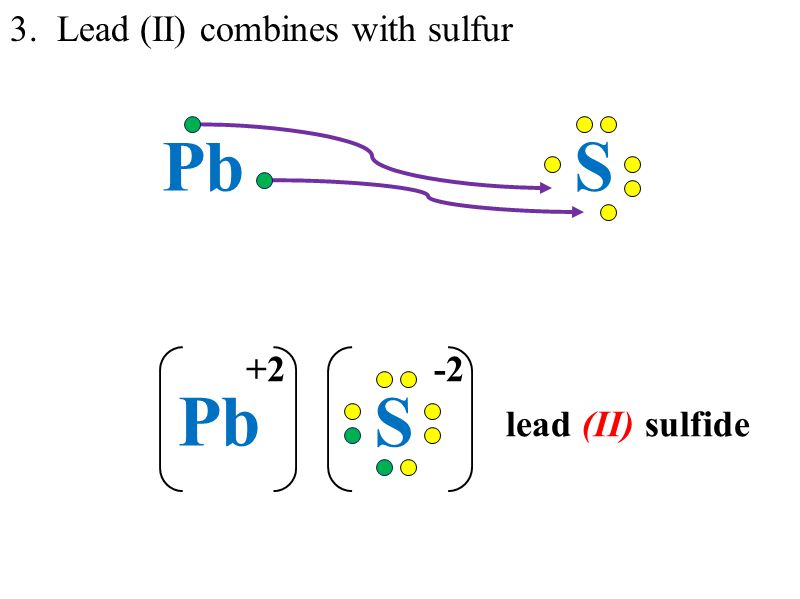
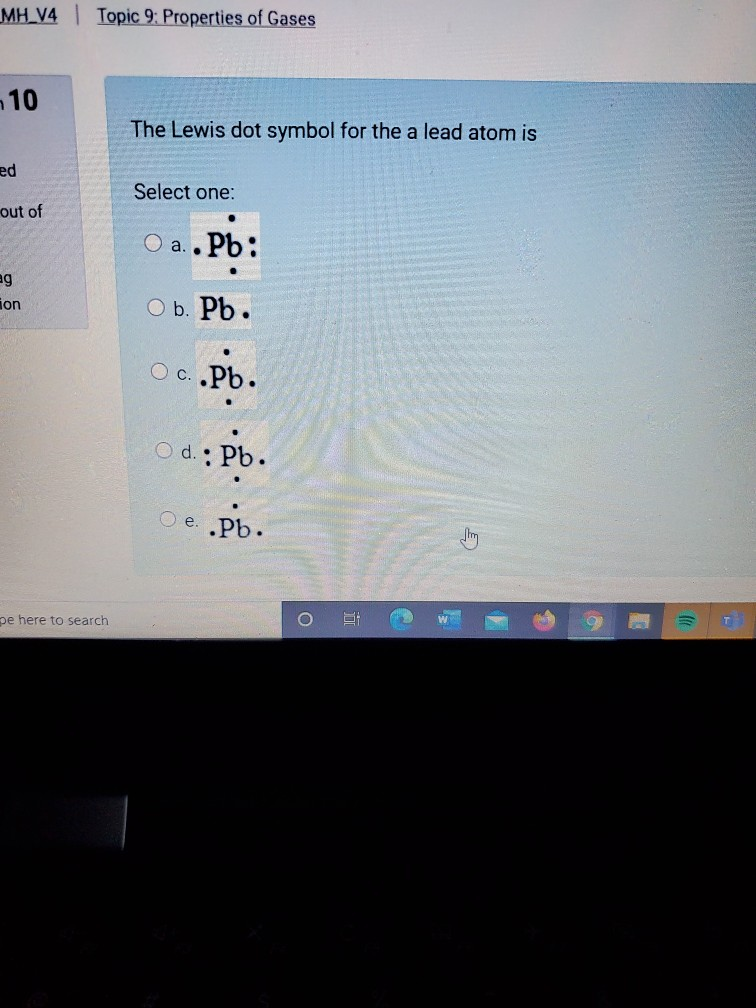


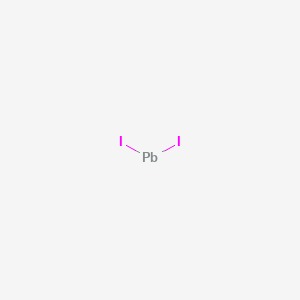
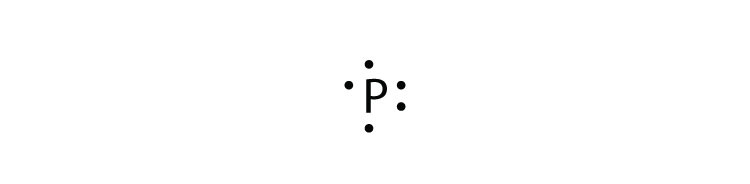

0 Response to "38 lewis dot diagram for lead"
Post a Comment