40 orbital diagram for silicon
Just so, what is the orbital diagram for silicon? Silicon has 14 electrons in the following orbital configuration 1s2 2s2 2p6 3s2 3p2 when neutral in charge. Also the crystalline form is used in semiconductors. Phosphorus 1s 2s 2p 3s 3p 4s 3d 4p. In writing the electron configuration for silicon the first two electrons will go in the 1s orbital. Orbital diagram of Silicon (Si) 15: Orbital diagram of Phosphorus (P) 16: Orbital diagram of Sulfur (S) 17: Orbital diagram of Chlorine (Cl) 18: Orbital diagram of Argon (Ar) 19: Orbital diagram of Potassium (K) 20: Orbital diagram of Calcium (Ca) 21: Orbital diagram of Scandium (Sc) 22:
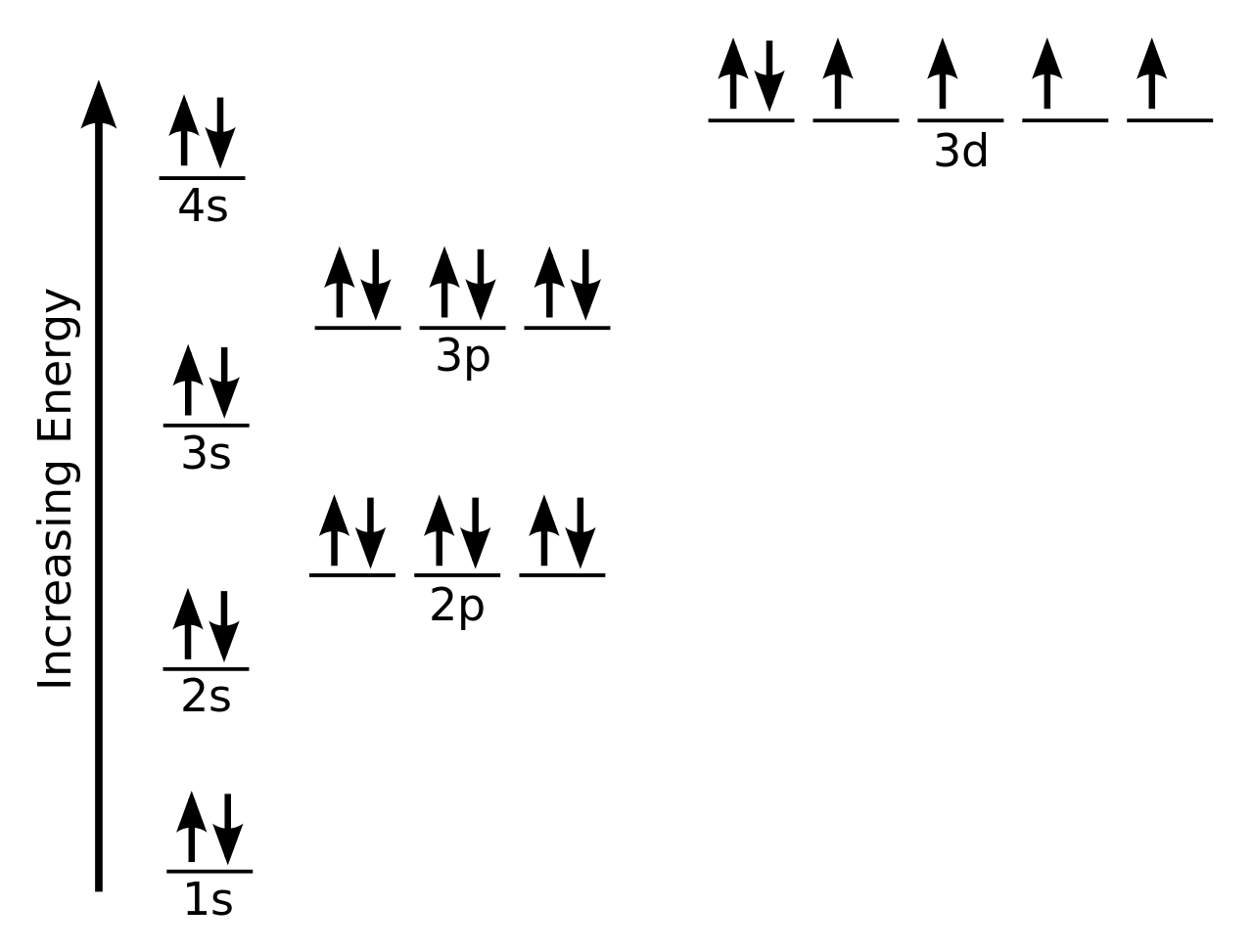
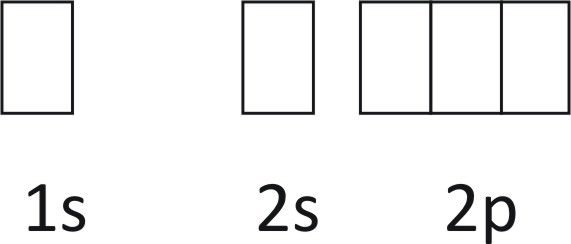
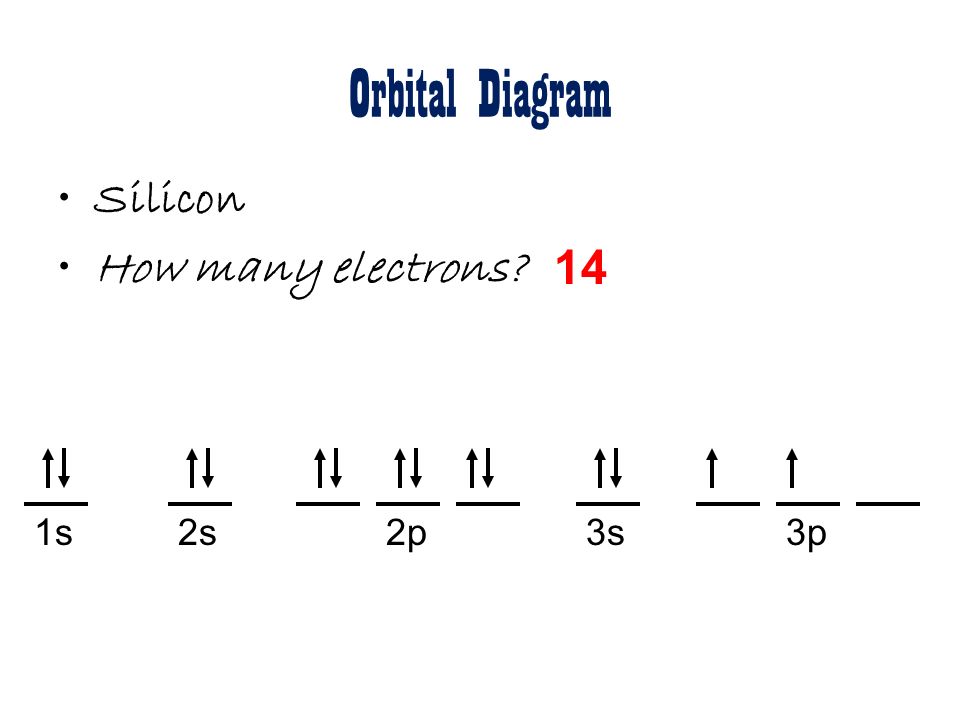
In writing the electron configuration for Silicon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Silicon go in the 2s orbital. The nex six electrons will go in the 2p orbital.

Orbital diagram for silicon
In writing the electron configuration for Silicon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Silicon go in the 2s orbital. The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two ... How Many Valence Electrons are in Silicon. There are 4 electrons in the outer shell of Silicon so the number of valence electrons in silicon is 4. Silicon Orbital Diagram. Orbit diagram consists of a pair of electrons of the atom in the box i.e. Orbit diagram helps to define the ground-state electron configuration is an easy form. 1s^2 2s^2 2p^6 3s^2 3p^2 When adding electrons, the lowest energy levels are always filled first. This is shown by the Aufbau princible shown here: The lowest energy level is the 1s. All orbitals hold two electrons, and there is one possible orbital for s electrons to have, so we add two electrons to 1s. The same is true for the next highest level, 2s. 2p is different because there are three ...
Orbital diagram for silicon. The Orbital Diagram for Silicon: The orbital diagram for an element shows the electron distribution of the electrons, and the correct pairing of electrons with respect to electron spin. Show the orbital-filling diagram for N (nitrogen). ... Give the ground-state electron configuration for silicon (Si) using noble-gas shorthand. [Ne]3s^23p^2. Item 3: Part C Give the actual ground-state electron configuration for copper (Cu) using the complete form. 1s^22s^22p^63s^23p^63d^104s^1. Chemistry Chemistry: Matter and Change The electron configuration of silicon needs to be written and the orbital diagram of silicon needs to be drawn applying the Pauli Exclusion Principle, the Aufbau principle, and Hund's rule. Concept introduction: Aufbau Principle states that in the ground state electrons of an atom fill the orbital with lowest energy first and upcoming electrons are filled ... This time we have collected a handy of orbital diagrams with various types in high definition! There are alkali, argon, electron, nitrogen, phosphorus, silicon, and sulfur orbital diagrams that you can save for free. Orbital diagrams are pictorial descriptions of the electrons in an atom. Three rules are useful in forming orbital diagrams.
Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics. The orbital filling diagram for helium. The electron configuration for helium is 1s². This means that we have two electrons in the 1s orbital, which looks like this: This diagram is exactly the same as the one for hydrogen, except that there's a second arrow added to the 1s orbital. This represents the second electron in the 1s orbital, and ... It is a metalloid that has the atomic number 14 in the periodic table. It is in Group 14 of the periodic table. It has the symbol Si. Silicon is the. 8th most abundant element in the universe and is the second most abundant element by weight on earth. It is most commonly found in compounds and never found naturally. Orbital Diagram For Arsenic. Because the 4p section has 3 orbitals, but Arsenic ends with 4p3. It'll want to leave as few orbitals empty, so you have three arrows pointing up. The orbital diagram of arsenic can be written as 1s2 2s2 2p6 3s23p6 4s2 3d10 4p3. Arsenic has 33 electrons, including 3 in itsoutermost shell. schematron.org!
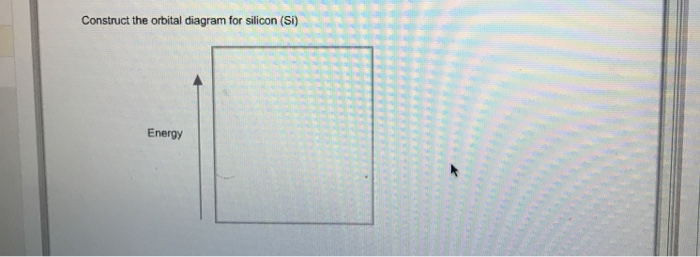
9.7 The orbital diagram below presents the final step in the CQ formation of hybrid orbitals by a silicon atom. (a) Do you think one or more electrons have been promoted? Why or why not? (b) What type of hybrid orbitals are being produced in this hybridization? [Section 9.5] 9.8 Consider the hydrocarbon drawn below. (a) What is the Orbital diagrams are pictorial descriptions of the electrons in an atom. Three guidelines are helpful in forming orbital diagrams. In accordance with the Auf Bau Precept, every electron occupies the bottom power orbital. You leap up somewhat bit in power and we get the 2s orbital that make it the 2p sublevel. Silicon Orbital Diagram Orbit diagram consists of a pair of electrons of the atom in the box i.e. Orbit diagram helps to define the ground-state electron configuration is an easy form. That is one box contains 2 electrons. And for silicon, there will be 7 box representations for 14 electrons in a pair. This problem has been solved! Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. plea …. View the full answer. Transcribed image text: Fill in the orbital energy diagram for silicon.
Orbital filling diagram for silicon. So you put 8 electrons into your energy level diagram. You can represent electrons as arrows. Also the crystalline form is used in semiconductors. If you havent yet learned electron configurations you really need to go ahead. Commercial production depends on a reaction between sand sio2 and carbon at a.
What is the orbital notation for silicon? Its hard to put this on a screen with a regular key board. But the 1's at the top represent arrows in the pared up arrows imagine the first one pointing ...
0 Comments. on Orbital Diagram For Germanium. orbital. Because an electron can have either one of two spins, any orbital can hold a maximum of four . The orbital diagram for germanium is. 1s. 2s. 2p. 3s. Oxidation States, +4,2. Electrons Per Shell, 2 8 18 4. Electron Configuration, [Ar] 3d10 4s2 4p2. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p2.
Orbital Diagram, electron configuration, and the noble gas notation for a silicon (Si) atom.
Electron configuration of Silicon atom (Fig. 2.1) The atomic number of Silicon atom is Z = 14. It contains 14 positive charges in the nucleus and 14 electrons that move about the nucleus in closed stationary orbits. The orbits are assumed to be concentric circles. Thus, each atom is electrically neutral (Zero charge for the atom as a whole).
Orbital diagrams are a visual way to show where the electrons are located within an atom. Orbital diagrams must follow 3 rules: The Aufbau principle, the Pau...
An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down arrows to represent the electrons in ...
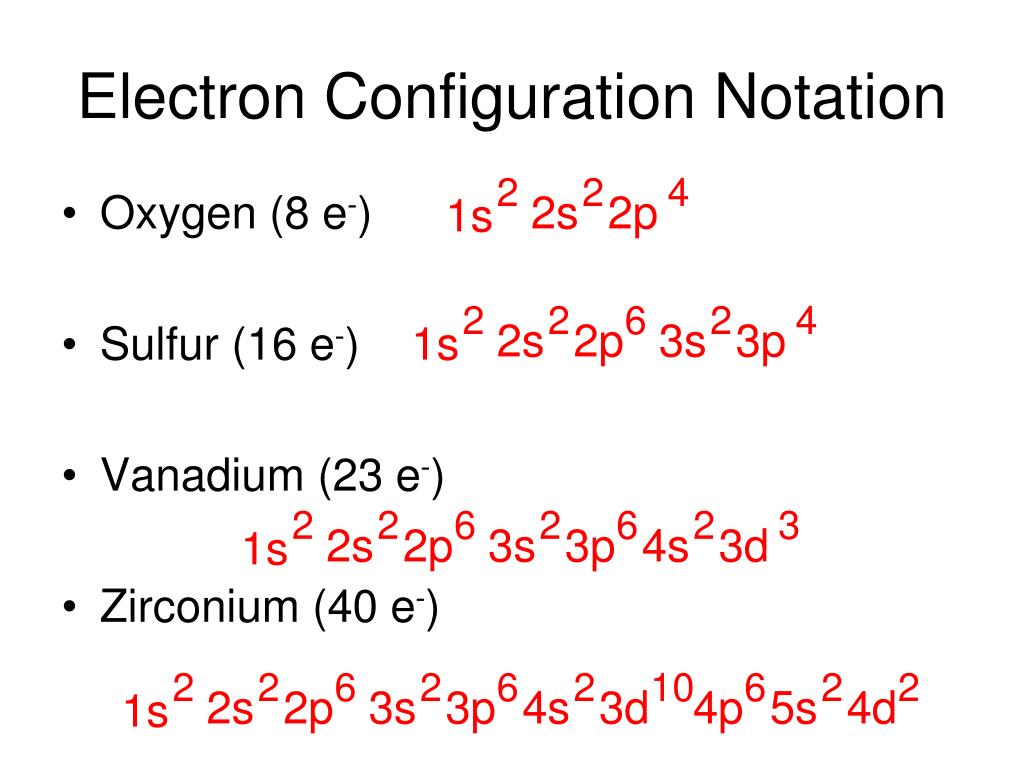
Silicon has an electron configuration of 1s2 2s2 2p6 3s2 3p2. Using the noble gas notation, the electron configuration of silicon can be denoted by Ne 3s 2 3p 2. In the periodic table of elements, silicon is represented by the chemical symbol Si, atomic number 14 and relative atomic mass of 28.085. It contains 14 protons and 14 electrons, with ...
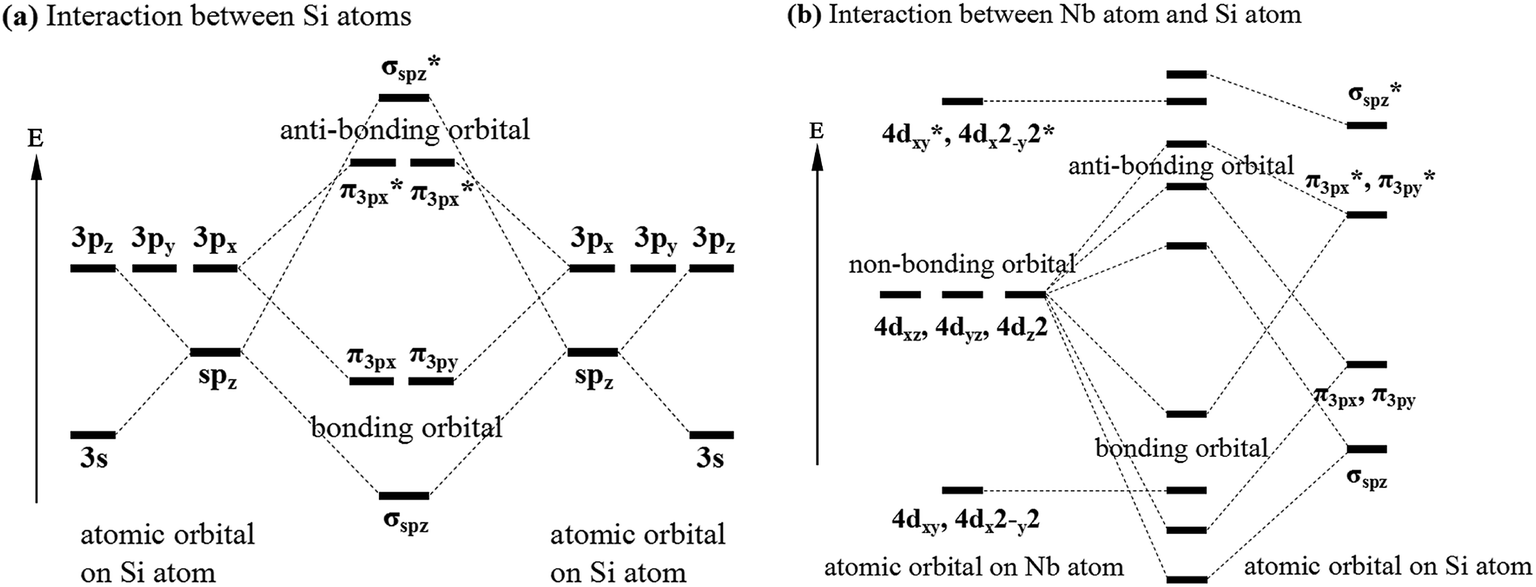
Figure 12.21 The Molecular Orbital Energy-Level Diagram for a Linear Arrangement of n Atoms, Each of Which Contains a Singly Occupied s Orbital. This is the same diagram as Figure 9.35 "Bonding in Ozone", with the addition of the far right-hand portion, corresponding to n = 30 and n = ∞. As n becomes very large, the energy separation between adjacent levels becomes so small that a single ...
Silicon orbital diagram. Show the orbital-filling diagram for S sulfur. It is a hard brittle crystalline solid with a blue-grey metallic lustre and is a tetravalent metalloid and semiconductorIt is a member of group 14 in the periodic table. Also the crystalline form is used in semiconductors. Three rules are useful in forming orbital diagrams.
Si orbital Diagram. electron configuration of silicon si orbital diagram orbital diagram electron configuration and the noble gas notation for a silicon si atom electron configuration for silicon si how to write the electron configuration for silicon si since 1s can only hold two electrons the next 2 electrons for silicon go in the 2s orbital.
1s^2 2s^2 2p^6 3s^2 3p^2 When adding electrons, the lowest energy levels are always filled first. This is shown by the Aufbau princible shown here: The lowest energy level is the 1s. All orbitals hold two electrons, and there is one possible orbital for s electrons to have, so we add two electrons to 1s. The same is true for the next highest level, 2s. 2p is different because there are three ...
How Many Valence Electrons are in Silicon. There are 4 electrons in the outer shell of Silicon so the number of valence electrons in silicon is 4. Silicon Orbital Diagram. Orbit diagram consists of a pair of electrons of the atom in the box i.e. Orbit diagram helps to define the ground-state electron configuration is an easy form.
In writing the electron configuration for Silicon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Silicon go in the 2s orbital. The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two ...
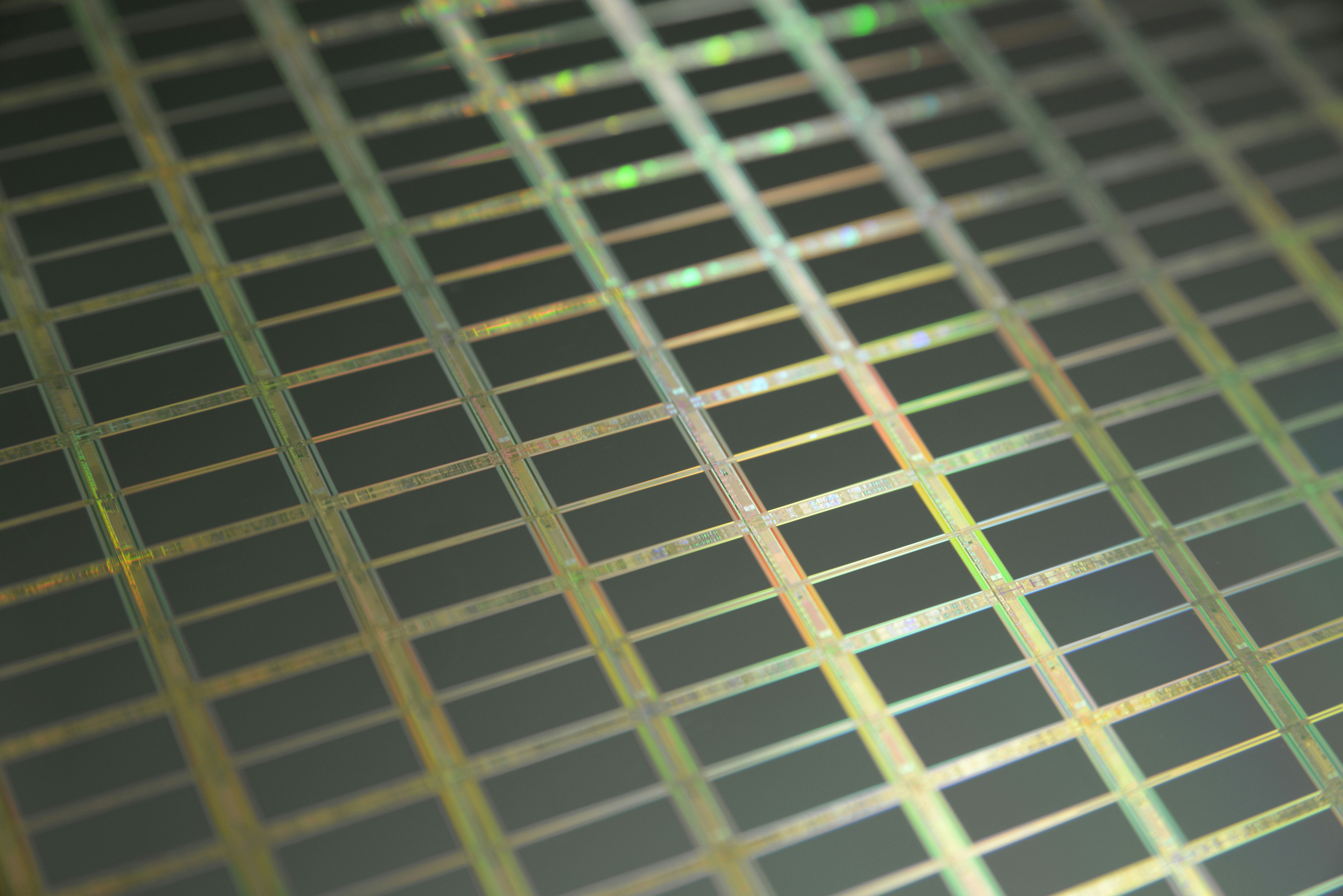
This is a macro of a silicon wafer. Each square is a chip with microscopic transistors and circuits. Ordinarily, wafers like these are diced into their individual chips and the chips go into the processors that power our computers. Sometimes, wafers have flaws and the manufacturers dispose of them instead. That’s how I got mine. After visiting the tech museums in Silicon Valley, I was amazed at the beauty of silicon wafers, so I started collecting and photographing them. Like fractals and flowers, the closer you get to them, the more amazing details there are to see.




















0 Response to "40 orbital diagram for silicon"
Post a Comment