41 the cell diagram for the lead-acid
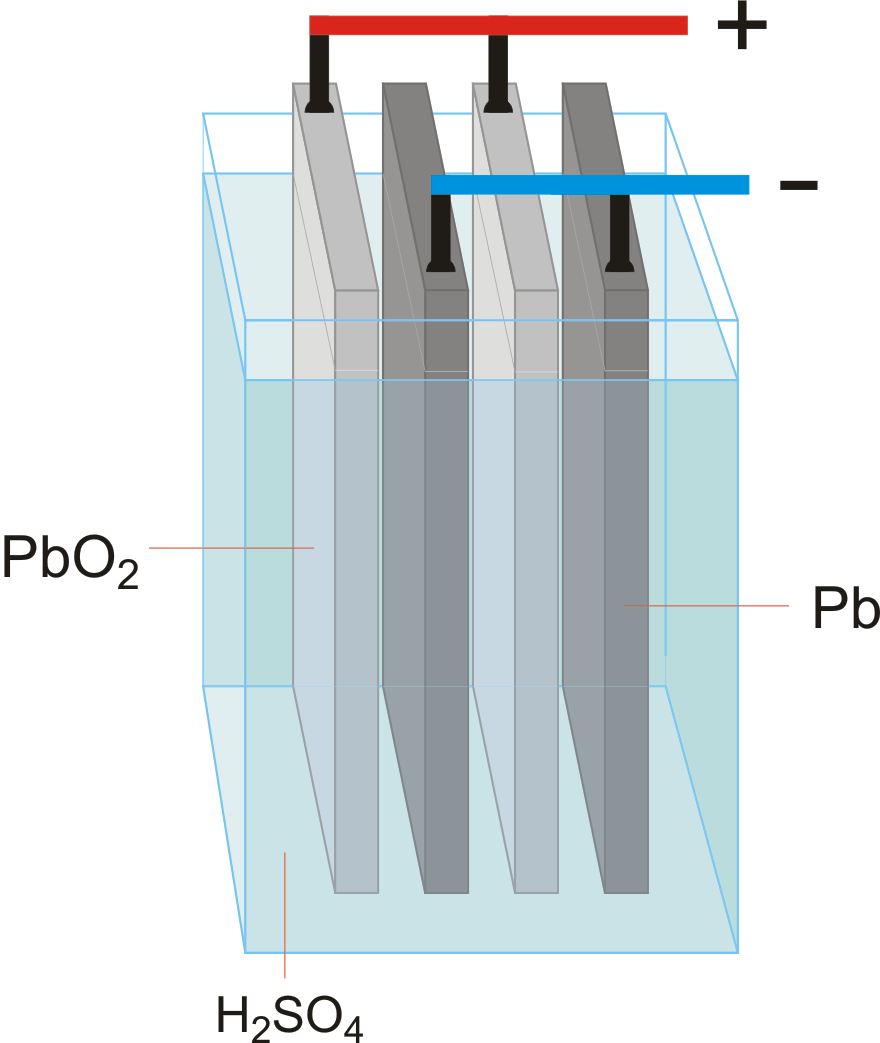
The cell diagram for the lead-acid cell that is used in automobile and truck batteries is Pb (s)|PbSO4 (s)|H2SO4 (aq)|PbO2 (s),PbSO4 (s)|Pb (s) The comma between PbO2 (s) and PbSO4 (s) denotes a heterogeneous mixture of the two solids. The right-hand lead electrode is nonreactive.
In this article we will discuss about the working of lead-acid battery with the help of diagram. When the sulphuric acid is dissolved, its molecules break up into hydrogen positive ions (2H +) and sulphate negative ions (SO 4 - -) and move freely.Now if two lead electrodes are immersed in this solution and connected to dc supply mains, the hydrogen ions being positively charged move ...
The cell diagram for the lead-acid cell that is used in automobile and truck batteries is. The comma between PbO2 (s) and )PbSO4 (s) denotes a heterogeneous mixture of the two solids. The right-hand lead electrode is nonreactive. Write the balanced equation for the net cell reaction. Look up standard potentials for the oxidation and reduction ...
The cell diagram for the lead-acid
In this topic, you study the definition, diagram and working of the lead acid battery and also the chemical reactions during charging and discharging. The combination of two or more than two cells suitably connected together is known as a battery. In case of lead acid cell, the cell has got the following parts. Parts of lead acid battery.
The cell diagram for the lead-acid cell that is used in automobile and truck batteries is Pb(s) l PbSO4 (s) l H2SO4 (aq) l PbO2(s), PbSO4(s) l Pb (s) where the comma between PbO2 (s) and PbSO4 (s) denotes a heterogeneous mixture of the two solids and the right-hand lead electrode is nonreactive. a)Write a balanced equation for the net cell reaction: b)Look up standard potentials for the ...
The hard plastic case is one cell. A single cell store typically 2.1V. Due to this reason, A 12V lead acid battery consists of 6 cells and provide 6 x 2.1V/Cell = 12.6V typically. Now, what is the charge storage capacity? It is highly dependable on the active material (Electrolyte quantity) and the plate's size.
The cell diagram for the lead-acid.
A Secondary Battery: The Lead Storage Battery. The electrodes of the cells in a lead storage battery consist of lead grids.The openings of the anodic grid is filled with spongy (porous) lead. The openings of the cathodic grid is filled with lead dioxide {PbO 2}.Dilute sulfuric acid {H 2 SO 4} serves as the electrolyte.When the battery is delivering a current, i.e.discharging, the lead at the ...
Sealed Lead Acid Battery. The sealed lead-acid battery consists of six cells mounted side by side in a single case. The cells are coupled together, and each 2.0V cell adds up to the overall 12.0V capacity of the battery.
difference across the cell terminals) of each cell will be about 2.1V, but this value will drop depending on how much current is being drawn. Six cells in series make up a twelve volt battery which when fully charged will have a terminal voltage of 12.6 to 12.8V. The EMF of lead-acid cells is dependent on
The cell diagram for the lead-acid cell that is used in automobile and truck batteries is Pb (s) l PbSO 4 (s) l H 2 SO 4 (aq) l PbO 2 (s), PbSO 4 (s) l Pb (s) where the comma between PbO 2 (s) and PbSO 4 (s) denotes a heterogeneous mixture of the two solids and the right-hand lead electrode is nonreactive.
Fuel cells and lead-acid batteries 10.626 (2011) Bazant. vertical lines. Reactions not involving H + such as (23) and (27) appear as horizontal lines. From reactions (28)-(29), we see that the Pb. 2+ ion is favored over metal-lic lead and lead oxide in acidic solutions and low potentials, and thus disso-
Plante plates or formed lead acid battery plates. Faure plates or pasted lead acid battery plates. Plante Plate Plante Process. In this process two sheets of lead are taken and immersed in dilute H 2 SO 4. When an current is passed into this lead acid cell from an external supply, then due to electrolysis, hydrogen and oxygen
Cell notation for the lead-acid battery. Bookmark this question. Show activity on this post. Reactions for the lead acid battery are: Oxidation P b ( s) + H S O X 4 X − ( l) P b S O X 4 ( s) + H X + ( l) + 2 e X − Reduction P b O X 2 + H S O X 4 X − ( l) + 3 H X + ( l) + 2 e X − P b S O X 4 ( s) + 2 H X 2 O Total reaction P b ( s) + P b ...
Lead-Acid battery storage are known to have slow performance at a low and high ambient temperature, as well as short life time (Morioka et al., 2001). A major setback for Lead-Acid battery storage system is that they require an infrequent water maintenance if flooding occurs, coupled with low specific energy of 30 Wh kg-1 and power of 180 W kg ...
Lead-Acid battery. Lead-acid battery is from the secondary galvanic cells, It is known as a Car battery (liquid battery) because this kind of batteries is developed and becomes the most suitable kind of batteries used in cars, It consists of six cells are connected in series, Each cell produces E cell = 2 volt and the total cell potential of the battery is emf = 2 × 6 = 12 volts, This battery ...
The cell diagram for the lead-acid cell that is used in automobile and truck batteries is Pb (s) l PbSO4 (s) l H2SO4 (aq) l PbO2 (s), PbSO4 (s) l Pb (s) where the comma between PbO2 (s) and PbSO4 (s) denotes a heterogeneous mixture of the two solids and the right-hand lead electrode is nonreactive.
Schematic Representation Of A Lead Acid Cell Scientific Diagram Maintenance Of Lead Acid Battery Electrical4u ... Schematic diagram of lead acid battery scientific a ilration the chemical reaction working secondary storage electrical4u construction and charging discharging more detailed drawing left hand batteries what is recharging circuit ...
Maintenance of lead acid cells : Care and maintenance of lead acid cells is most impotent. Other wise the batteries will get spoiled. The following points should be observed : 1)If the voltage of the lead acid cell comes down up to 1.8 volts per cell, the battery should not be used. It should be kept aside.
State two applications of lead acid storage cell. Last Answer : Applications of lead acid storage cell: 1. Lead acid storage cellis commonly used in automobiles. 2. To Supply current for electric vehicles 3. In Gas engine ignition 4. In telephone exchanges ... hospitals 7. In broad casting stations, power stations 8. For distribution work 9.
The right-hand lead electrode is nonreactive. Write the balanced equation for the net cell reaction. equation: Look up standard potentials for the oxidation and reduction half-reactions, and then calculate the value of 𝐸∘cellEcell∘. 𝐸∘cell=Ecell∘=. VV. Calculate the value of Δ𝐺∘rxnΔGrxn∘. Δ𝐺∘rxn=ΔGrxn∘=.
Lead-Acid Battery Construction. The lead-acid battery is the most commonly used type of storage battery and is well-known for its application in automobiles. The battery is made up of several cells, each of which consists of lead plates immersed in an electrolyte of dilute sulfuric acid. The voltage per cell is typically 2 V to 2.2 V.
As the lead-acid cell discharges: PbSO 4 precipitates out and deposits on both the anode and the cathode.; H + from the electrolyte (H 2 SO 4(aq)) is being used to produce water at the cathode.; Concentration of H + will be decreased over time (concentration of H 2 SO (aq) decreases).; pH of the electrolyte (H 2 SO 4(aq)) will increase.; Connecting lead-acid galvanic cells in a series to make ...
A lead-acid battery is the most inexpensive battery and is widely used for commercial purposes. It consists of a number of lead-acid cells connected in series, parallel or series-parallel combination. A lead-acid cell basically contains two plates immersed in electrolyte (dilute sulphuric acid i.e. H 2 SO 4 of specific
The cell diagram for the lead-acid cell that is used in automobile and truck batteries is Pb (s) l PbSO4 (s) l H2SO4 (aq) l PbO2 (s), PbSO4 (s) l Pb (s) where the comma between PbO2 (s) and PbSO4 (s) denotes a heterogeneous mixture of the two solids and the right-hand lead electrode is nonreactive.
These larger crystals are unlike the typical porous structure of the lead electrode, and are difficult to convert back into lead. Voltage of lead acid battery upon charging. The charging reaction converts the lead sulfate at the negative electrode to lead. At the positive terminal the reaction converts the lead to lead oxide.
Lead Acid Battery. Definition: The battery which uses sponge lead and lead peroxide for the conversion of the chemical energy into electrical power, such type of battery is called a lead acid battery. The lead acid battery is most commonly used in the power stations and substations because it has higher cell voltage and lower cost.
The cell diagram for the lead-acid cell that is used inautomobile and truck batteries is Pb(s) l PbSO4 (s) l H2SO4 (aq) l PbO2(s), PbSO4(s) l Pb (s) where the comma between PbO2 (s) and PbSO4 (s) denotes aheterogeneous mixture of the two solids and the right-hand leadelectrode is nonreactive. a)Write a balanced equation for the net cell reaction:
A lead-acid cell is a basic component of a lead-acid storage battery (e.g., a car battery). A 12.0 Volt car battery consists of six sets of cells, each producing 2.0 Volts. A lead-acid cell is an electrochemical cell, typically, comprising of a lead grid as an anode
difference (usually measured in volts) is commonly referred to as the voltage of the cell or battery. A single lead-acid cell can develop a maximum potential difference of about 2 V under load. A completely discharged lead-acid cell has a potential difference of about 1.75 V, depending on the rate of discharge. Capacity and Battery Ratings

0 Response to "41 the cell diagram for the lead-acid"
Post a Comment