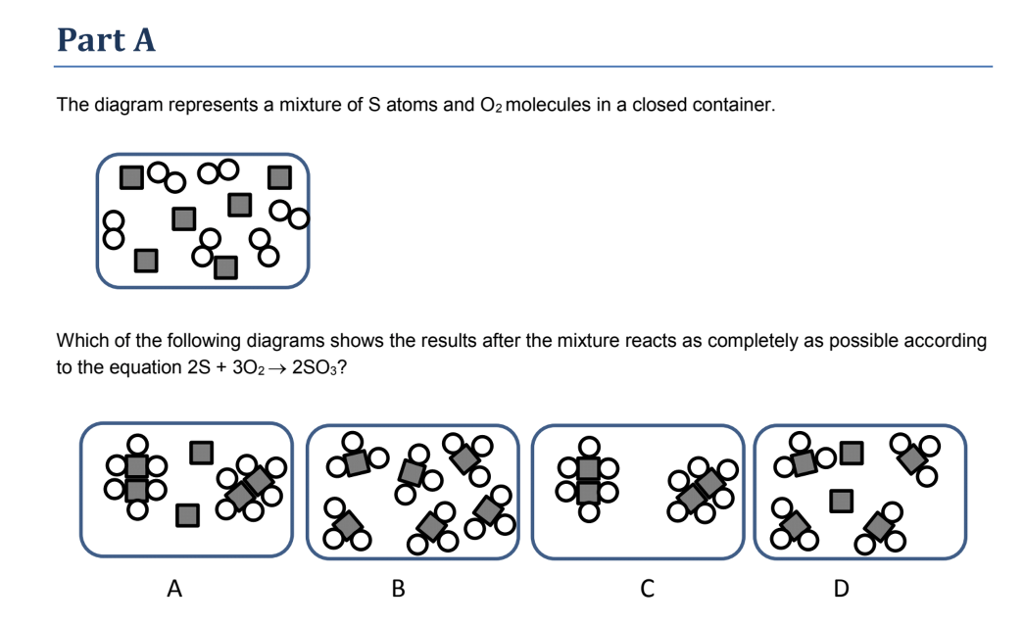
41 diagram of a mixture
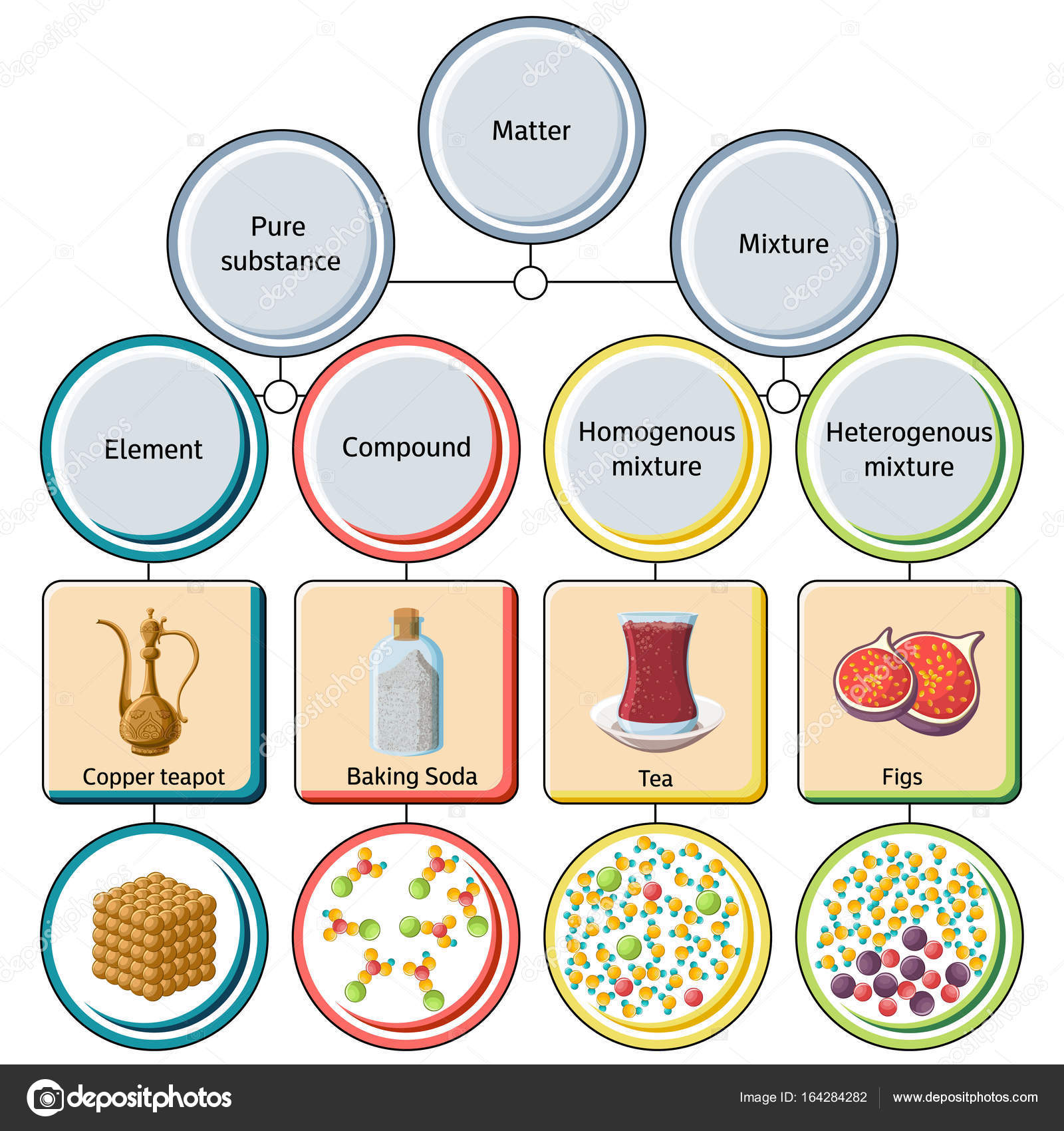
A mixture of sand mixed with salt is an example of a heterogeneous mixture. Heterogeneous mixtures possess different properties and compositions in various parts i.e. the properties are not uniform throughout the mixture.
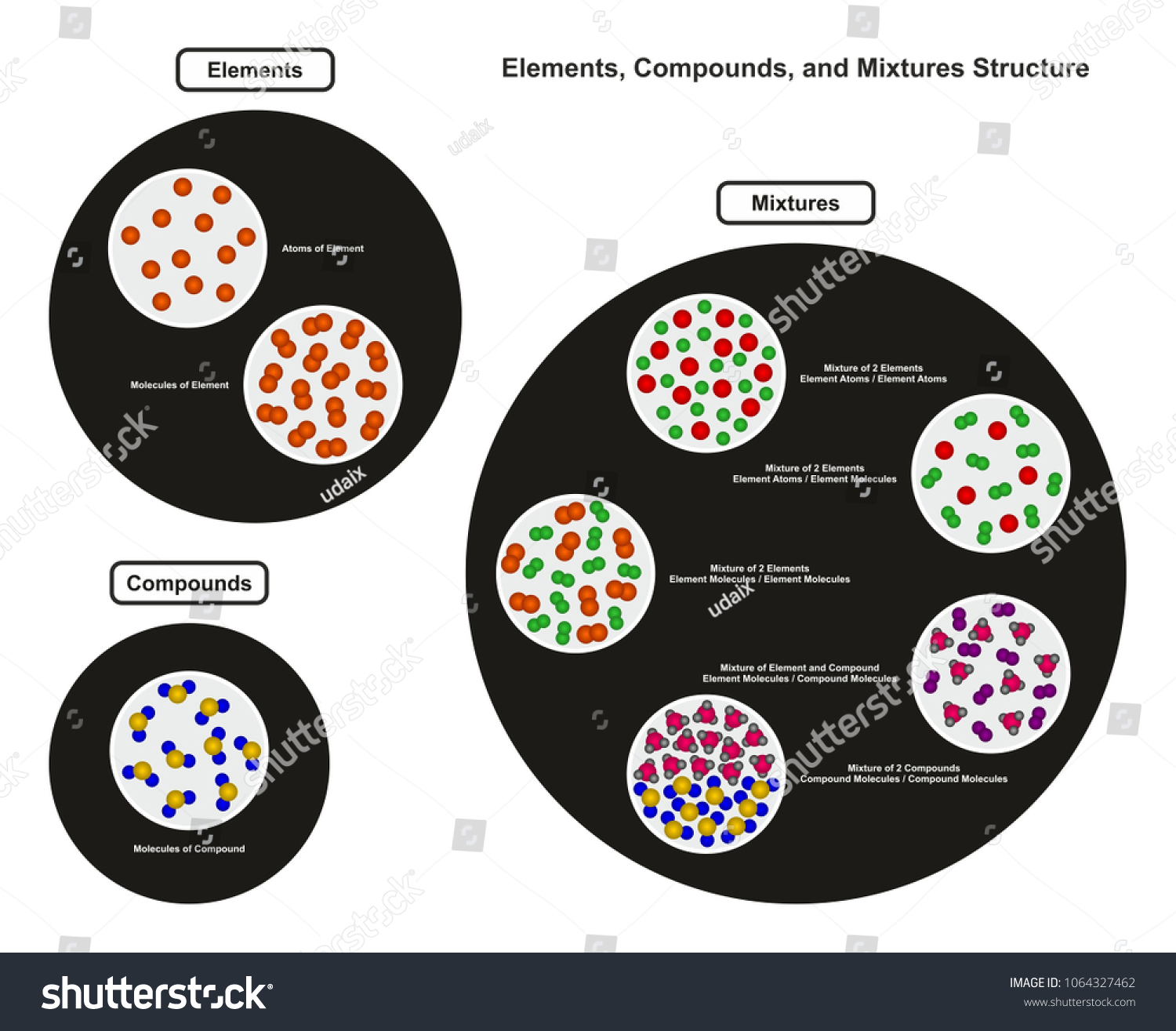
Mixture of two compounds - two types of compounds present. _D_5. Mixture of a compound and an element. Part 4: Column A lists a substance. In Column B, list whether the substance is an element (E), a compound (C), a Heterogeneous Mixture (HM), or a Solution (S). (Remember a solution is a homogeneous mixture.) In Column C, list TWO physical
What is a Mixture? Mixtures are the substances composed of two or more forms of matter. You can separate them by physical methods. Such examples include a mixture of salt and water, a mixture of sugar and water, different gases, air, etc. In any mixture, the various components do not form through any kind of chemical changes.
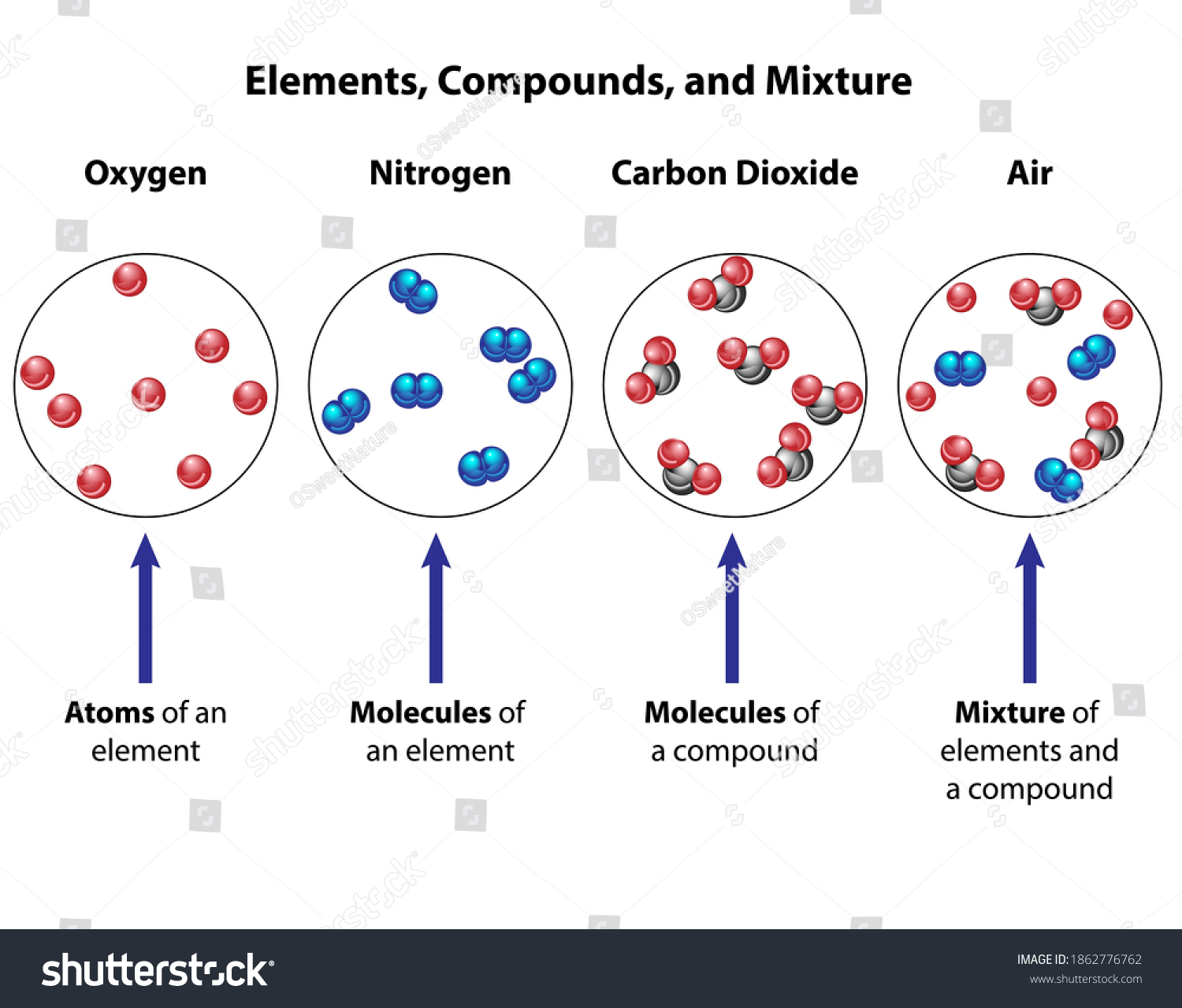
Diagram of a mixture
Learn to plot T-xy, P-xy and y-x diagram for a binary mixture using Aspen Plus. Objective:1. Learn how to plot Txy, Pxy, xy diagram of mixture2. Require inpu...
Phase diagrams methanol mixtures. Figure 7.2 A three-dimensional phase diagram for a Type I binary mixture (here, CO2 and methanol). The shaded volume is the two-phase liquid-vapor region. This is shown ti uncated at 25 °C for illustration purposes. The volume surrounding the two-phase region is the continuum of fluid behavior.
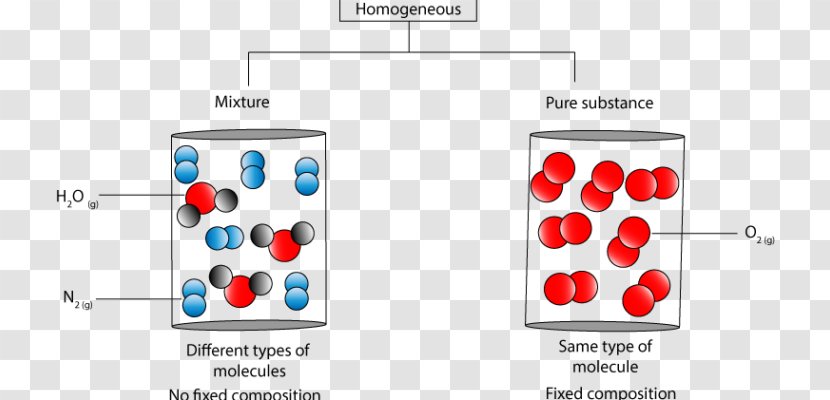
A heterogeneous mixture is a type of mixture that allows the components to be seen as two or more phases are present. A mixture is an example of water. Water is a homogeneous mixture of nitrogen, oxygen and smaller amounts of other compounds in the gaseous materials. Stay tuned with BYJU'S to learn more interesting topics in Chemistry.
Diagram of a mixture.
Describes how to use an interactive simulation that plots pressure versus temperature for a mixture of ethane and hexane. This simulation is located at: ht...
phase diagram, the alloy will become fully solid at the eutectic temperature, shown as the eutectic isotherm on the phase diagram. Construction: The region below the eutectic isotherm, and outside the solid solution region, will be a solid mixture of alpha and beta, that is, a two phase region, and the diagram is labeled to reflect this.
A mixture of acetone and chloroform shows this behavior in the bottom part of Figure 4.1-1. The Pxy diagram is plotted at 328 K and the Txy diagram is plotted at 1 atm. The data for vapor pressure and Wilson model are from the Thermosolver program by Koretsky. This program can also plot Pxy and Txy diagrams for different mixtures.
- Mixtures - more than one phase • Solubility Limit : Max concentration for which only a single phase solution occurs. Question: What is the solubility limit at 20°C? Answer: 65 wt% sugar . If Co < 65 wt% sugar: syrup If Co > 65 wt% sugar: syrup + sugar. 65 Sucrose/Water Phase Diagram Pure Sugar Temperature (°C) 0 20 40 60 80 100
The boiling point diagram shows how the equilibrium compositions of the components in a liquid mixture vary with temperature at a fixed pressure. Consider an example of a liquid mixture containing 2 components (A and B) – a binary mixture. This has the following boiling point diagram.
Carburetor - Diagram , working , parts ,types. Carburetion: The process of preparing a combustible fuel-air mixture outside engine cylinder in SI engine is known as carburetion. Important factors which affect the process of carburetion are given below; -time available for the mixture preparation i.e. atomisation, mixing and the vaporization.
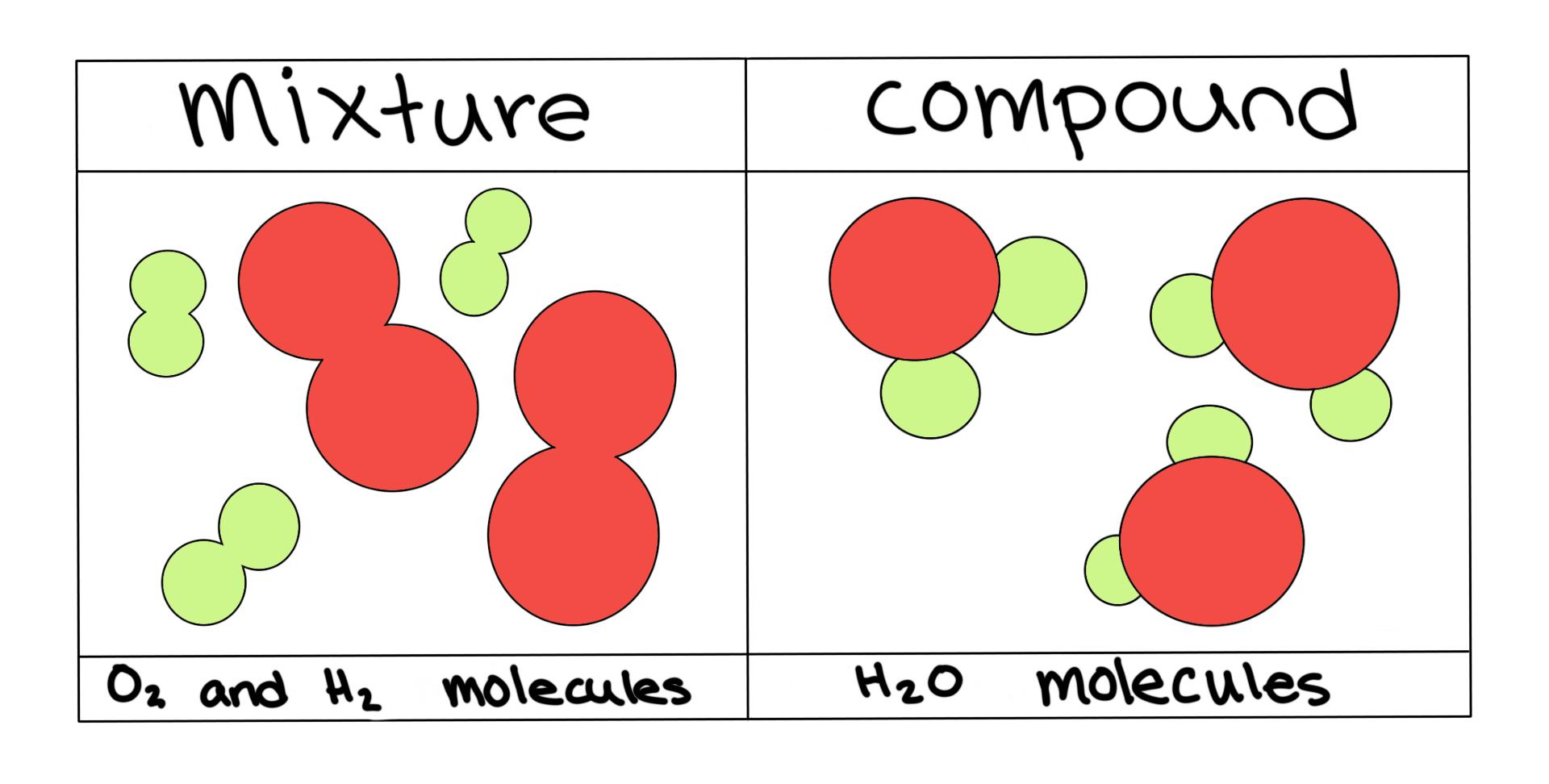
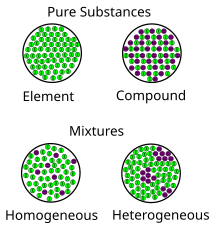
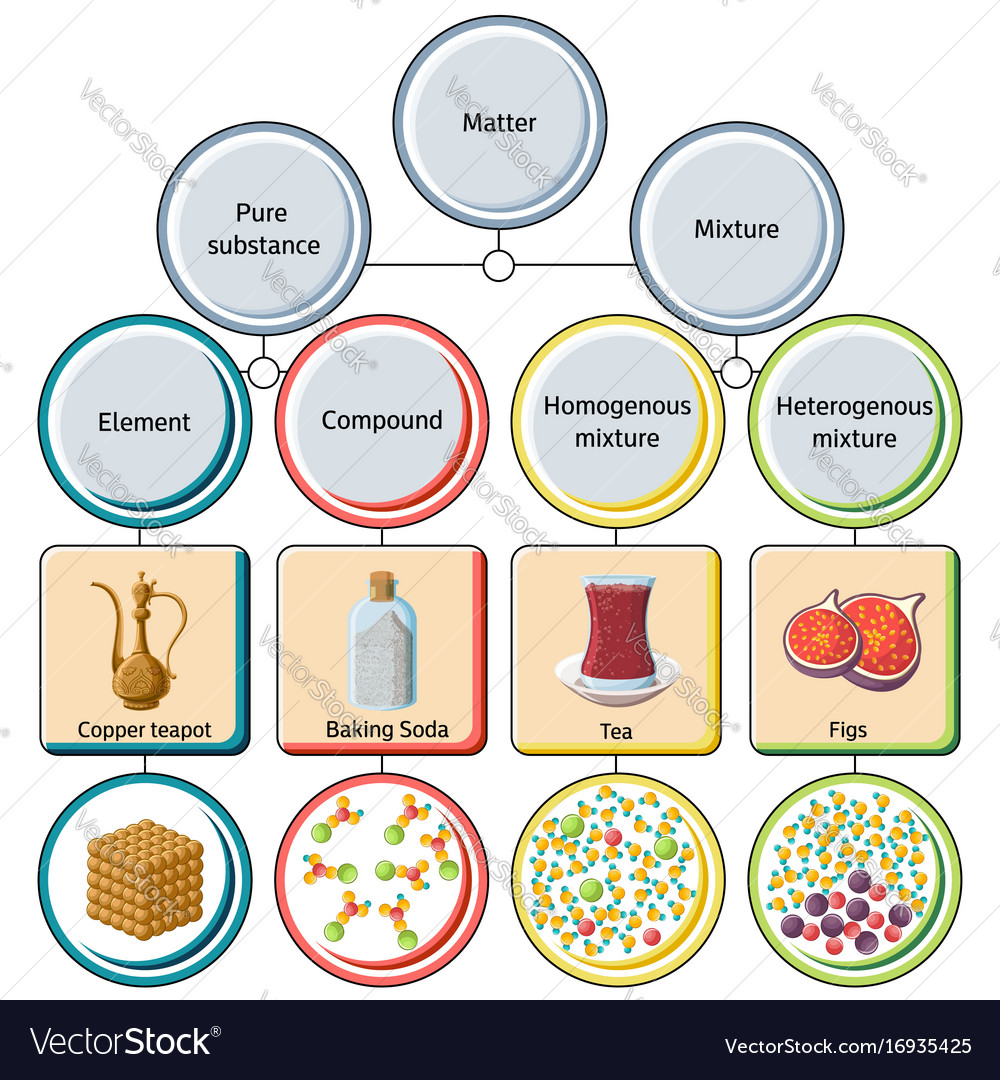
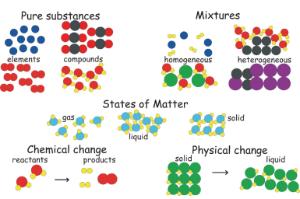
mixture. A combination of two or more substances that are not chemically combined. compound. A substance made up of atoms of two or more different elements joined by chemical bonds. pure substance. A sample of matter, either a single element or a single compound, that has definite chemical and physical properties (one type of particle) element.
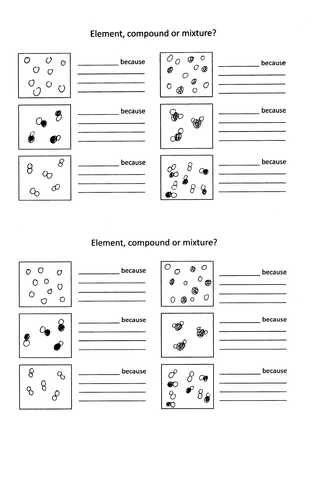
A mixture contains more than one type of atom or molecule. The following diagrams show the molecules in two pure substances before mixing, and the mixture of molecules afterwards. Look at the diagrams closely, and label each of them as either a single substance, or a mixture. 1. _____ 2. _____ 3. _____ Elements, compounds and mixtures (2 ...
For binary mixture phase diagram only two-component mixture, (e.g. A (more volatile) and B (less volatile)) are considered. There are two types of phase diagram: constant pressure and constant temperature. 5.1.3. Constant Pressure Phase Diagram The Figure 5.1 shows a constant pressure phase diagram for an ideal solution ...
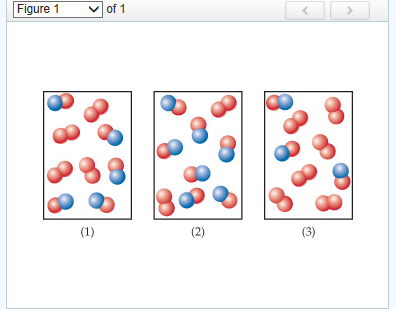
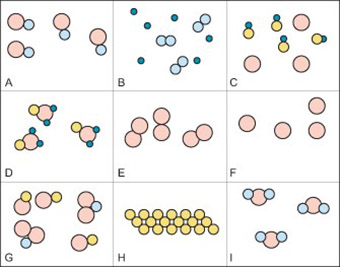
julie. Answered 5 months ago. A, because noble gases are monatomic which leaves us with A and D, but D is not a mixture since it only contains 1 kind of gas. Upvote · 4.
These 3-component mixtures show coexisting bilayer phases over much of the composition space. A standard format for showing the phase behavior of all possible combinations of a 3-component mixture is the triangular phase diagram, or "Gibbs Triangle".
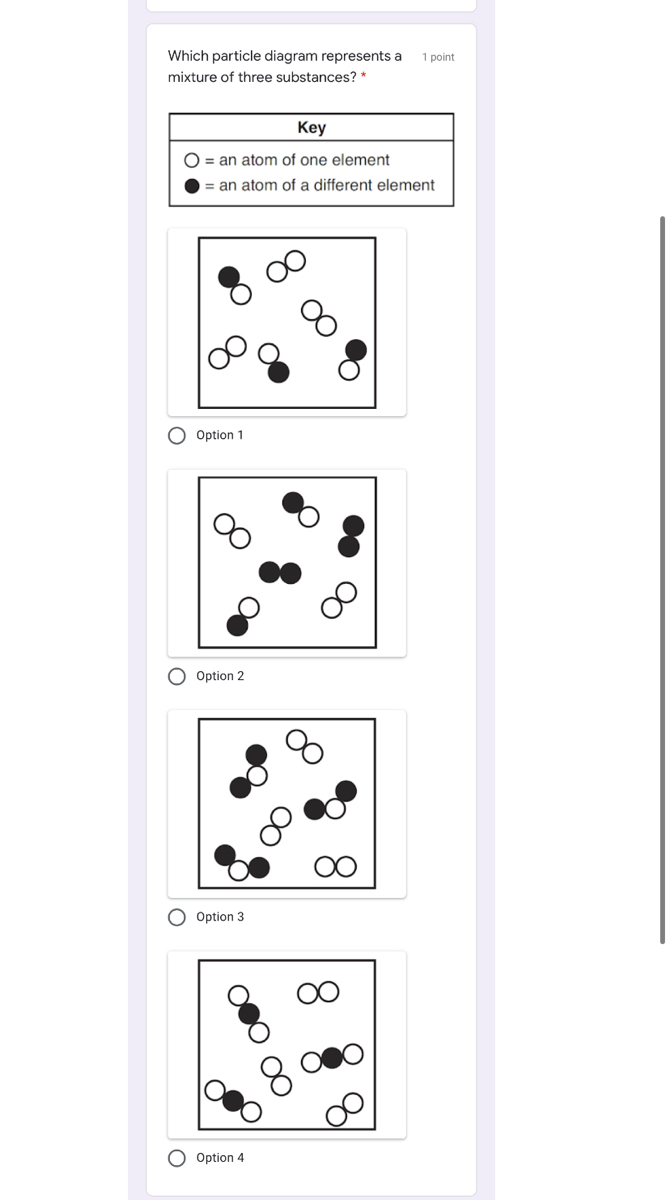
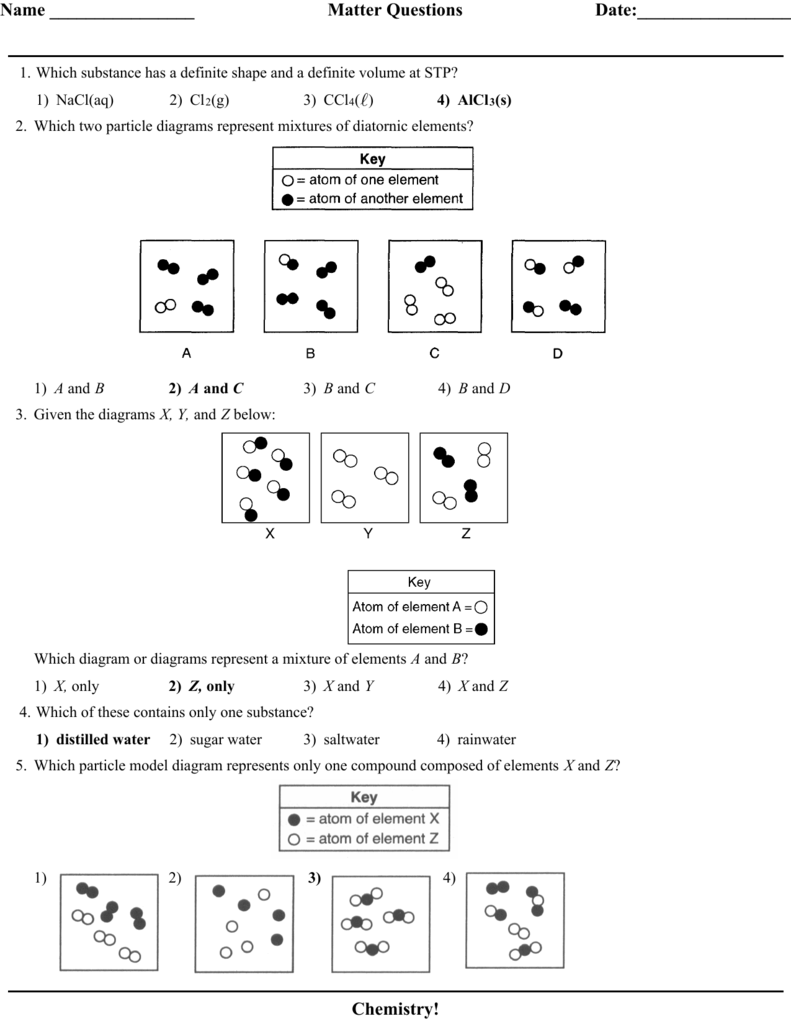
1.Which two particle diagrams represent mixtures of diatomic elements? A) mass B)density C) length D) volume 2.At STP, which physical property of aluminum always remains the same from sample to sample? A) CO2(aq) B) CO2(g) C) CO2( ) D)CO2(s) 3.Which sample of CO2 has a definite shape and a
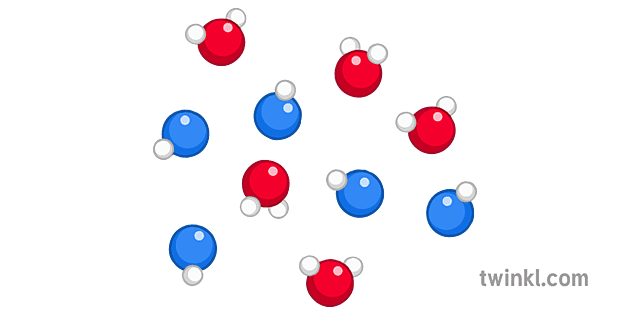
In an ideal mixture of these two liquids, the tendency of the two different sorts of molecules to escape is unchanged. You might think that the diagram shows only half as many of each molecule escaping - but the proportion of each escaping is still the same. The diagram is for a 50/50 mixture of the two liquids.
Chapter 8 Phase Diagrams. (b) The interpretation of diagrams. Point a represents the vapor pressure of a mixture with liquid composition xA and b represents the composition of the vapor that is in equilibrium with the liquid at that pressure. Note that when two phases are in equilibrium, P = 2, so F’ = 1.
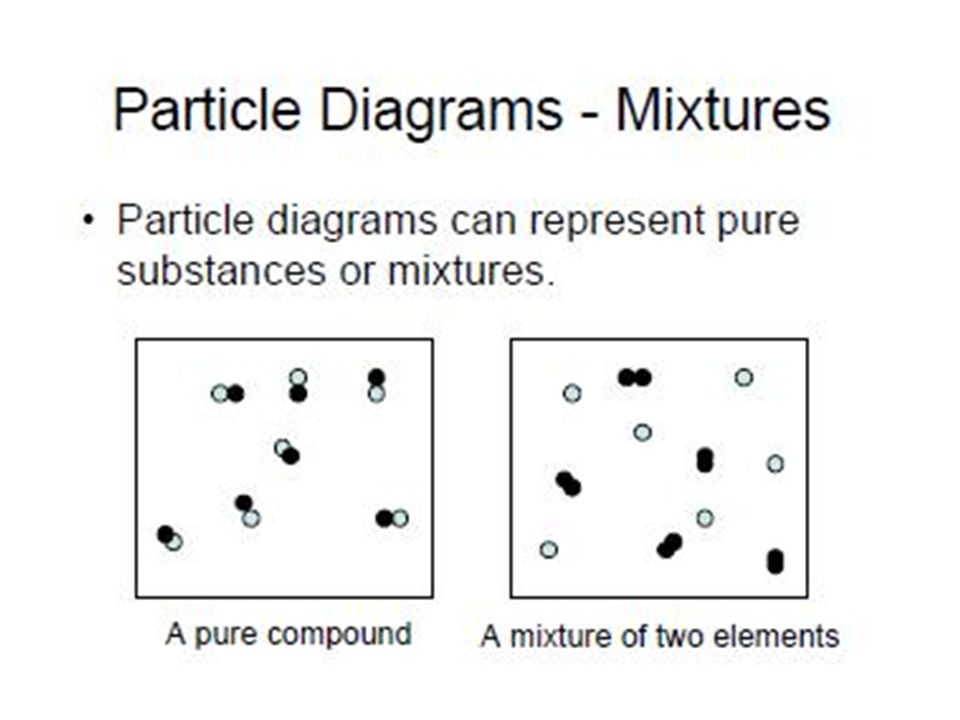
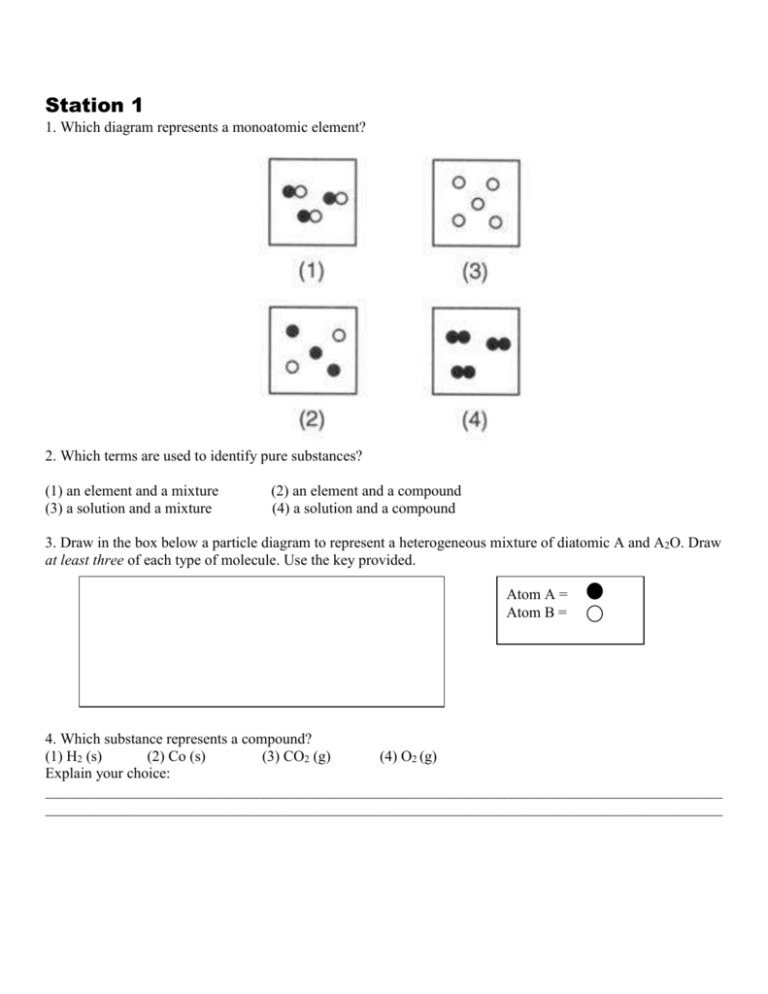
diagrams. •A particle diagram is a box in which coloured balls are draw to represent atoms or molecules. •These diagrams can represent elements and compounds, as well as their molecular composition by the types of balls and how they are connected. Element Compound Mixture of an Element and Compound
Short Tutorial on Using Spreadsheet to Obtain Txy Diagrams (T.B.Co 10/29/2001) Introduction. The calculations for bubble point temperature and dew point temperature of an ideal binary mixture usually require numerical methods to aid in determining the required values.
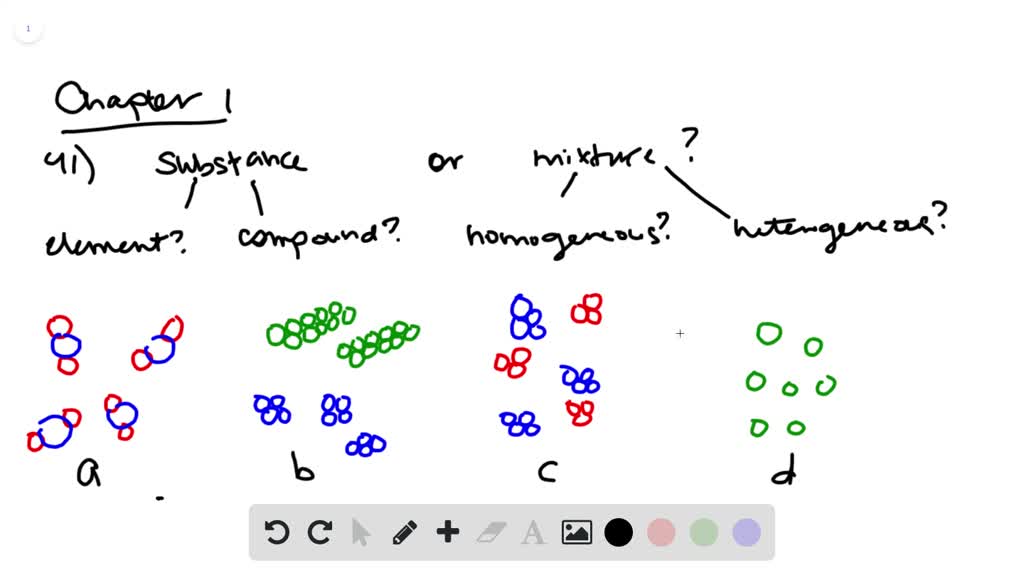
Determine whether each molecular diagram represents a pure substance or a mixture if it represents a pure substance classify the substance as an element or a compound if it represents a mixture classi
Experimental Determination of 2-Component Phase Diagrams. As an example, we're going to look at how one might go about determining the stability of a mixture of 2 mineral phases, A and B. To perform these experiments we start with pure minerals A and B and then make mixtures in varying proportions.
Definition: The P-xy and the T-xy are diagrams that represent the liquid and vapour equilibrium for a binary mixture. The component that is graphed is the most volatile one because is the one that will evaporate first during the distillation process. On the x-axis goes the mole fraction x,y (for liquid phase and vapour phase) and the y-axis is the temperature if its a P-xy or the pressure if its a T-xy.
and Particle Diagrams Aim: To represent the different forms of matter using particle diagrams. CLASSIFICATION OF MATTER SUBSTANCES ELEMENTS and COMPOUNDS HOMOGENEOUS and HETEROGENEOUS MIXTURES Composition is variable. Composition is in fixed proportions. Diatomic Element-Found only combined in
The following diagram shows how magnetic separation can be used to separate a mixture of components. In the example, mineral ore that contains two compounds (one magnetic, and the other non-magnetic) is being separated.
Ans: An eutectic point is the lowest melting temperature for a mixture that can be obtained from the phase diagram indicating the chemical composition of any such mixture. From the phase diagram, the temperature that is the lowest melting point temperature from the different melting points of different components of the mixture is known as the eutectic point.
We call the temperature at which a mixture begins to boil (when the first bubble of vapor forms) the bubble point temperature (or pressure) of the mixture. The bubble point as a function of composition is shown on a T xy diagram as the line forming the bottom of the phase envelope.
mixture. The infinite slope at cA=0 and 1 means that it is very hard to remove final few impurities from a mixture. AM Donald 3 Phase Diagrams This is the situation if no molecular interactions to ... construct a phase diagram Πwhich contains all the essential physics.





















0 Response to "41 diagram of a mixture"
Post a Comment