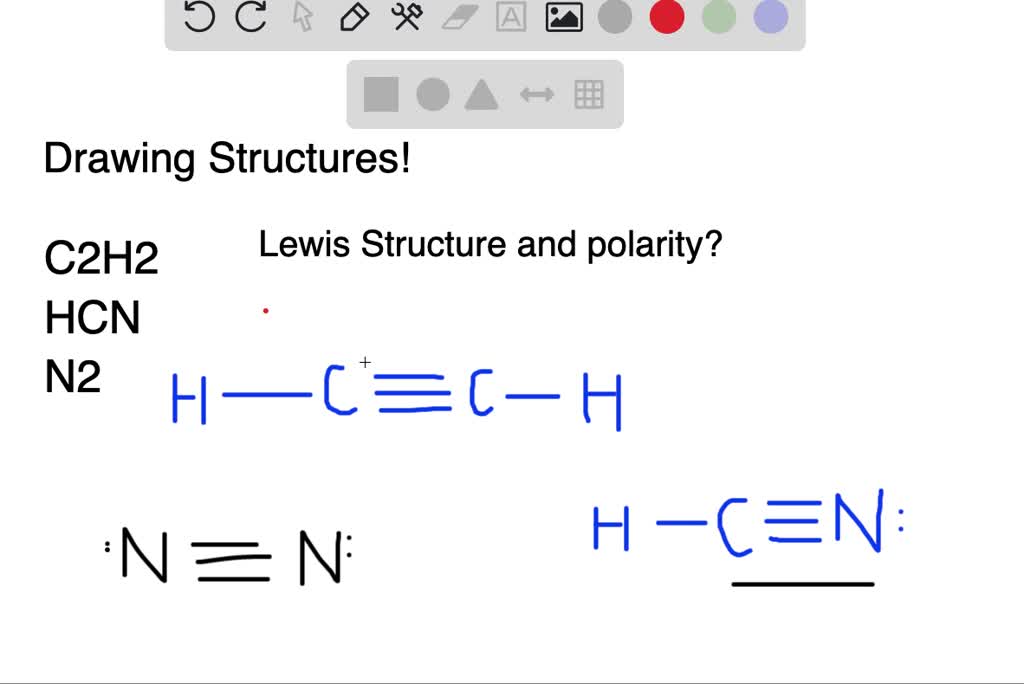
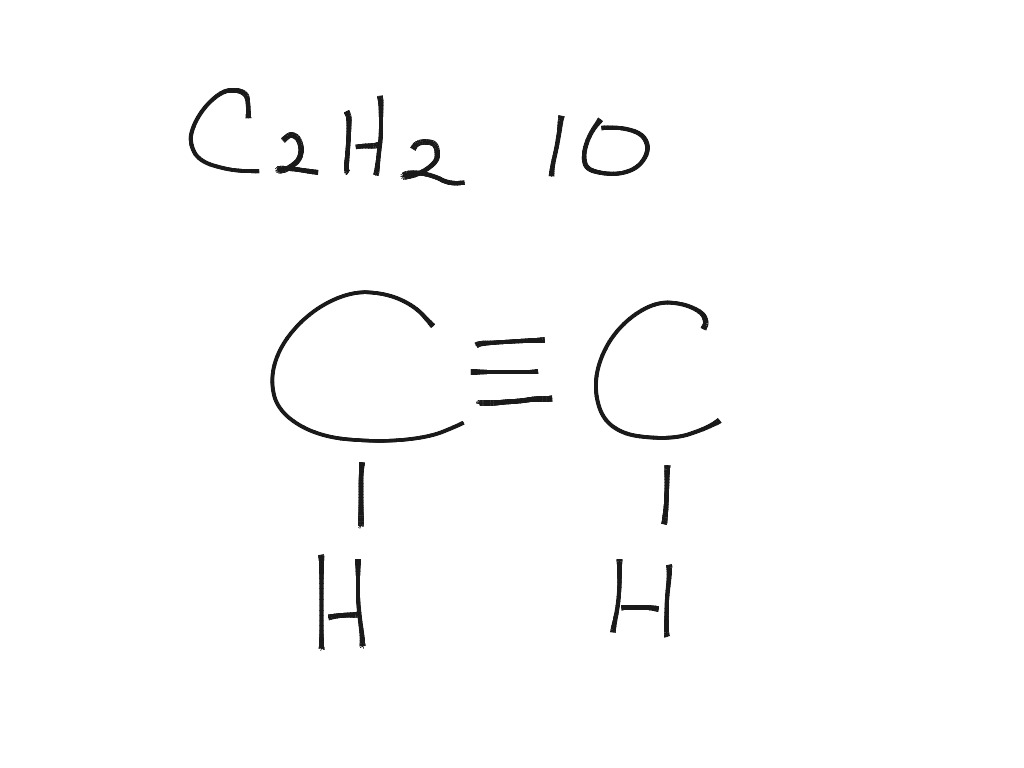
41 lewis diagram for c2h2
General, Organic &. Biological Chemistry (1st Edition) Edit edition Solutions for Chapter 4 Problem 9P: The Lewis structure for acetylene (C2H2) is drawn as Explain why it is possible to answer YES to the three questions posed in Sample Problem 4.2 for this Lewis structure … Lewis Structure For C2h2 C2H2 Lewis Structure Tutorial How to Draw the Lewis Lewis Dot Structure of C2H2 or CHCH Acetylene or ethyne Organic chemistry. The solution is to share three pairs of valence electrons and form a triple bond between the Carbon atoms in C 2 H 2. Final Exam Review - Chemistry with Coccine at.
Acetylene was found in whole gas octane levels 87, 89 and 92 at 0.0022, 0.0032 and 0.0037 ppbC%, respectively (1). Acetylene has been detected 12%, 2.9% and 50% of total hydrocarbon concentration in emissions from vehicle exhaust, petroleum exhaust and petrochemical plants, respectively (2).
Lewis diagram for c2h2
Get the free "Lewis structure" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha. A quick explanation of the molecular geometry of C2H2 including a description of the C2H2 bond angles. Note, the Hydrogen atoms (H) should not have lone pair... A step-by-step explanation of how to draw the C2H4 Lewis Dot Structure (Ethene).For the C2H4 structure use the periodic table to find the total number of val...
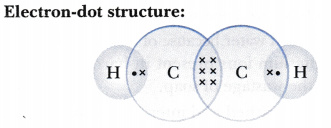
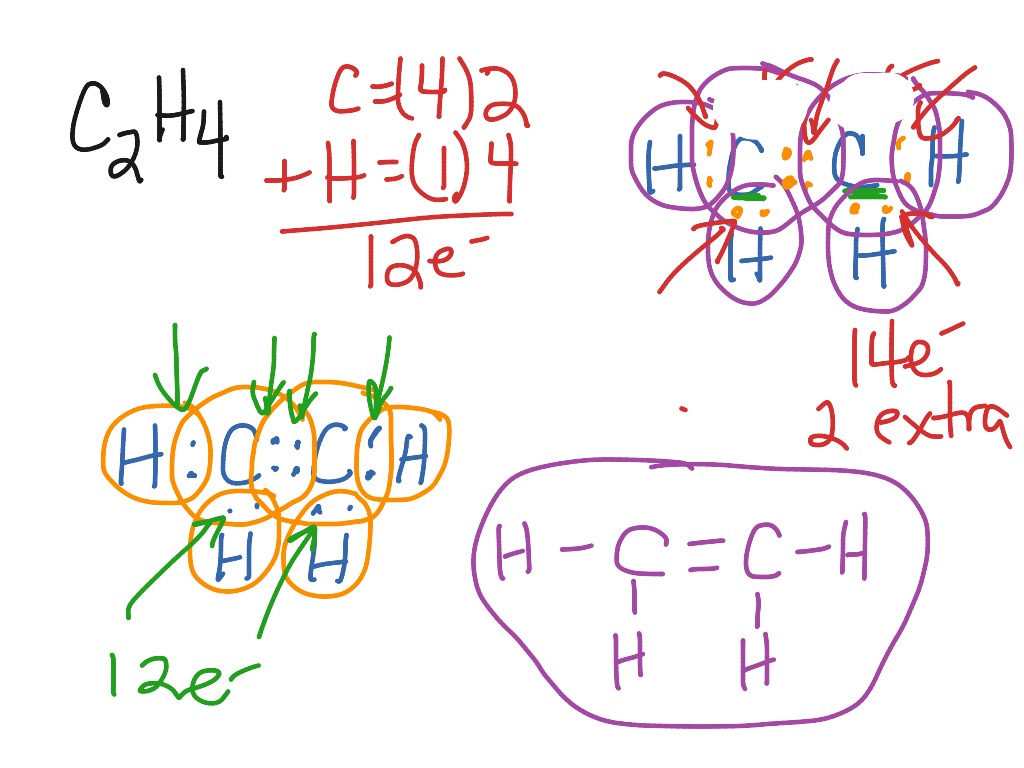
Lewis diagram for c2h2. C2H6 Lewis Structure. Lewis structure is a 2D representation of the compound, which represents only the valance shell electrons of the atoms in the molecule. It is based on the octet rule i.e. every atom tends to complete its octet ( 8 electrons) either by gaining or losing electrons except Hydrogen and Helium as they complete their duplet. Chemistry questions and answers. a.) Draw Lewis structures for the fluoroethene molecule (C2H3F), the acetonitrile molecule (CH3CN), and the acetylene molecule (C2H2) in the window below, and then answer the questions that follow, based on your drawings. Draw one structure per sketcher box. Separate added sketchers with + signs from the dropdown. C2H2 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO Diagram Acetylene or C2H2 is the simplest alkyne and a hydrocarbon that is colorless and has a garlic-like odor. It is highly reactive to atmospheric temperature and lacks oxygen being an unsaturated compound due to the presence of two carbon atoms bonded with a triple bond. C2H2 Lewis structure, Molecular Geometry, Hybridization & Bond angle. C2H2 is a chemical formula for Ethyne, a gaseous alkyne hydrocarbon. It has been used widely for welding and cutting purposes. This molecule is also known by the name Acetylene. The compound has a simple structure and is made up of two carbon atoms and two hydrogen atoms.
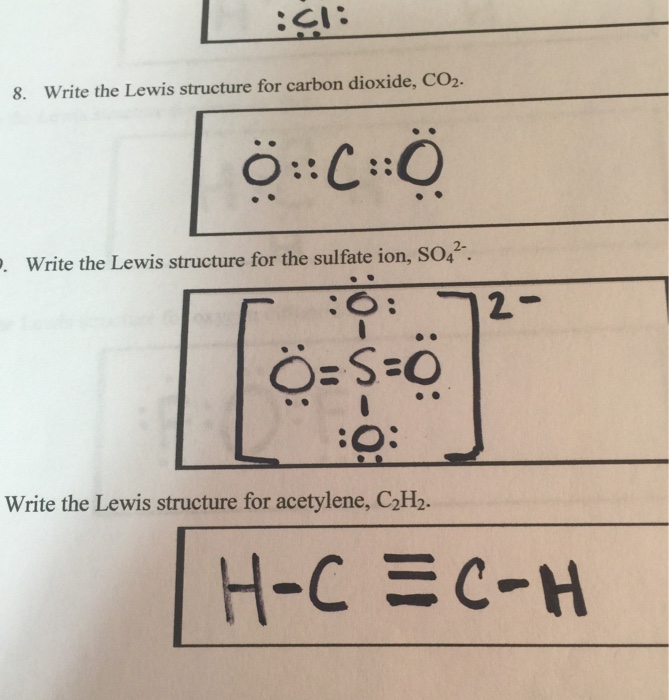
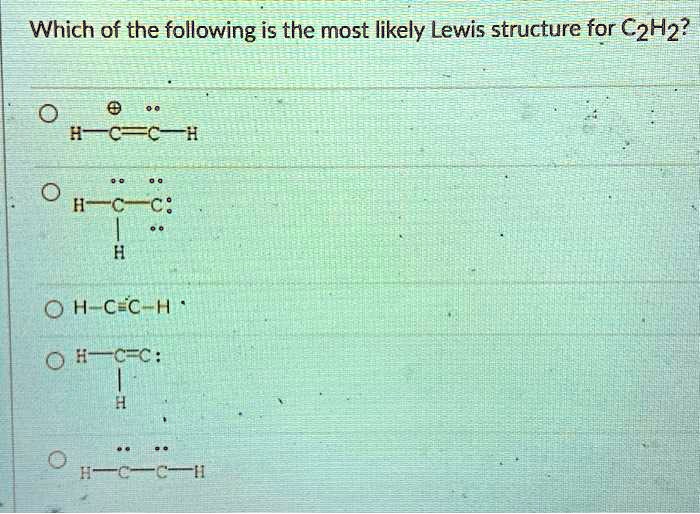
The correct Lewis structure for a molecule of the compound C2H2 contains an alkyne bond. This is a triple bond between two C atoms. It has a linear bond geometry and no lone pairs. 14+ C2H2 Lewis Structure. Which set of bonds is arranged in order of increasing polarity? (a) ph3 (b) ascl3 (c) sih4 (d) sbcl3 (e) h2s. Use lewis structures to show this sharing. Please help me with this lewis structure: Source: study.com. C2h2 would turn into what is known as ethyne. Source: i.ytimg.com. Drawing the Lewis Structure for C2H2 (Ethyne or Acetylene) For C2H2 you might have a complete of 10 valence electrons to work with. In drawing the Lewis structure for C2H2 (additionally known as ethyne) you will discover that you do not have sufficient valence electrons out there to fulfill the octet for every component (should you use solely ... Transcribed image text: Draw the Lewis structure of ethyne (C2H2) and then choose the appropriate pair of molecular geometries of the two central atoms. Your answer choice is independent of the orientation of your drawn structure. 5 L > Draw the Lewis structure of hydronium (H2O+) and then determine its electron domain and molecular geometries. 6 L > 0 of 1 point earned III O < Draw the Lewis ...
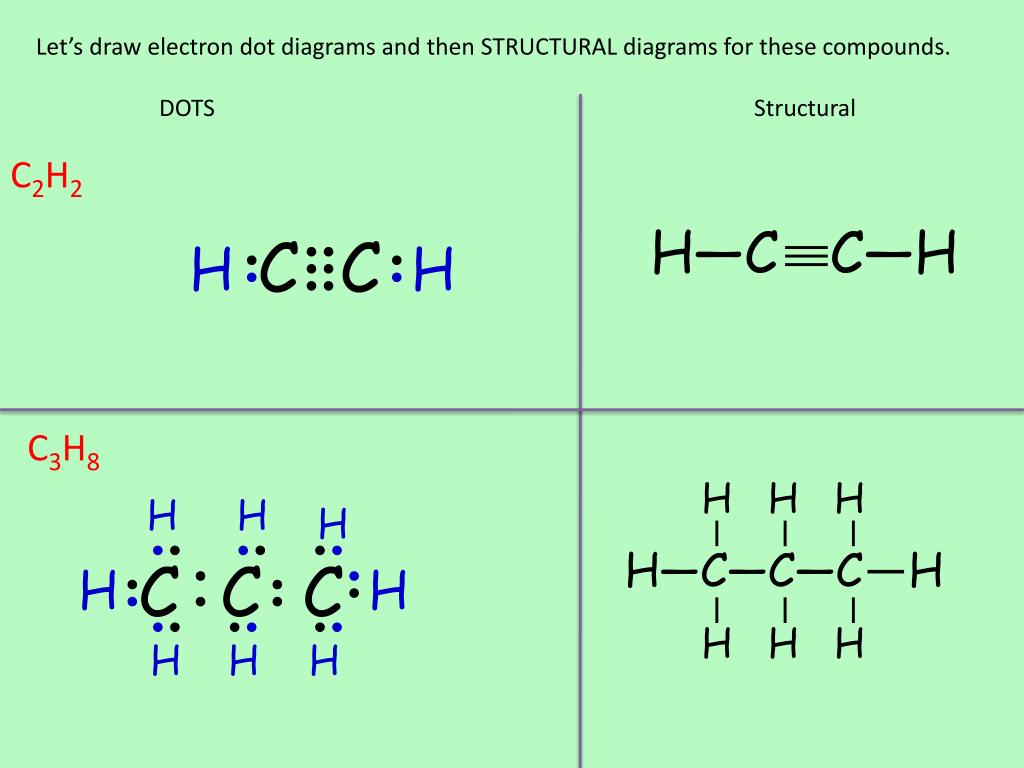
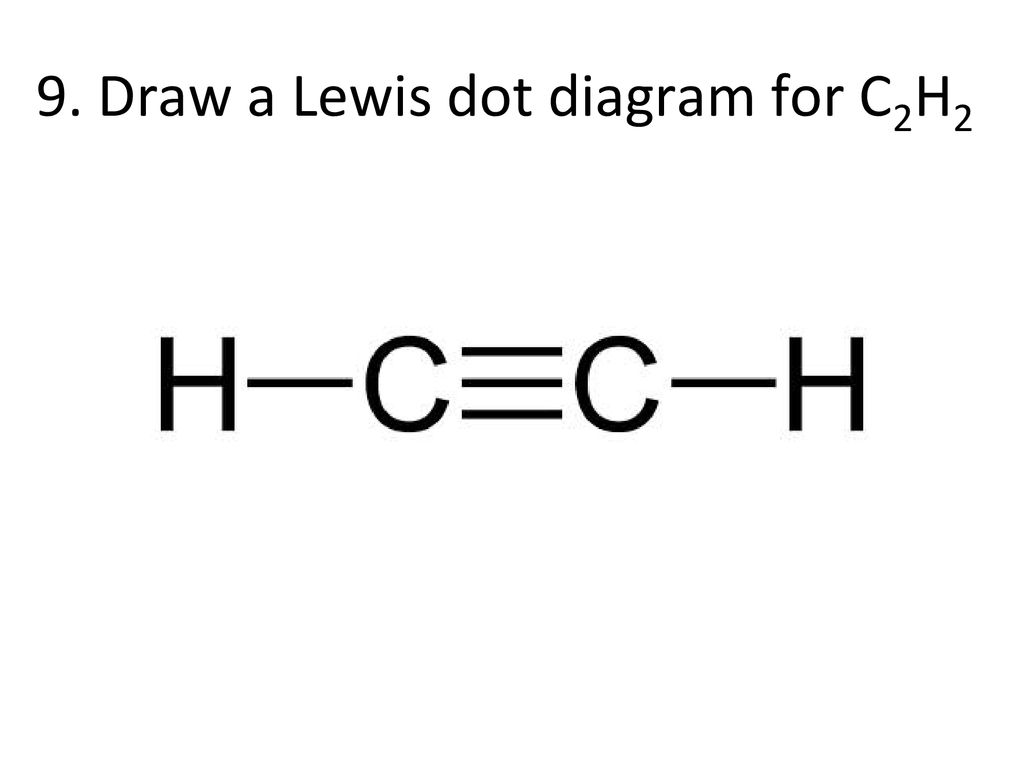
A step-by-step explanation of how to draw the C2H2 Lewis Dot Structure (Ethyne or Acetylene).For the C2H2 structure use the periodic table to find the total ... C2H2 (Acetylene | Ethyne) Lewis Structure. C2H2 (acetylene or ethyne) contains two carbon atoms and two hydrogen atoms. There is a triple bond between carbon atoms and hydrogen atoms are joint with carbon atoms though sigma bonds. There are no lone pairs on carbon or hydrogen atoms. In drawing the Lewis structure for C2H2 (also called ethyne) you'll find that you don't have enough valence electrons available to satisfy the octet for each element (if you use only single bonds). The solution is to share three pairs of valence electrons and form a triple bond between the Carbon atoms in C2H2 . Draw Lewis structures for acetylene molecule (C2H2), the chloroethane molecule (C2H5Cl), and the ethylene molecule (C2H4) in the window below, and then answer the questions that follow. a) give the bond order for the carbon carbon bond: C2H2. C2H5Cl. C2H4.
Lewis structure for c2h2. Since each line represents 2 shared electrons the carbon atoms share 8 electrons. Total valence electron Group Valence Electrons. In drawing the Lewis structure for C 2 H 2 also called ethyne youll find that you dont have enough valence electrons available to satisfy the octet for each element if you use only single bonds.
Drawing the Lewis Structure for C 2 H 2 (Ethyne or Acetylene). For C 2 H 2 you have a total of 10 valence electrons to work with.. In drawing the Lewis structure for C 2 H 2 (also called ethyne) you'll find that you don't have enough valence electrons available to satisfy the octet for each element (if you use only single bonds). The solution is to share three pairs of valence electrons and ...
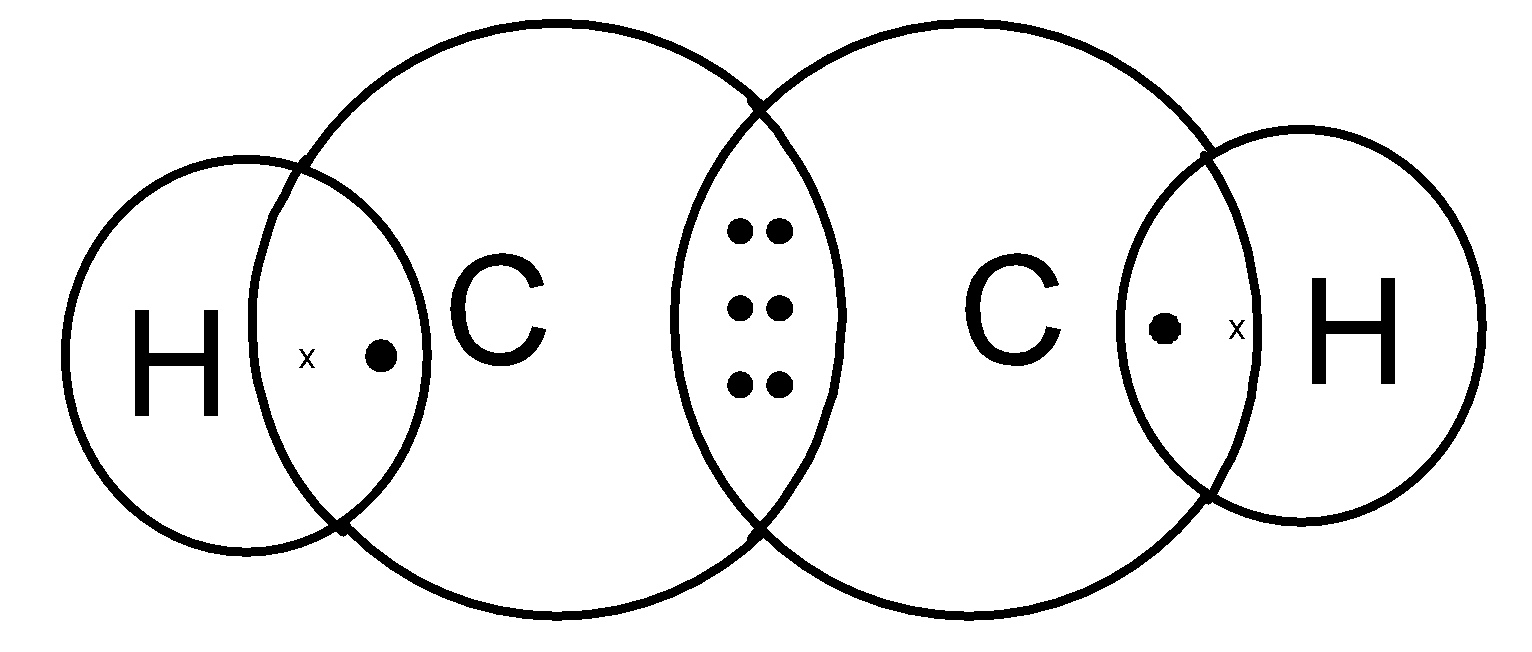
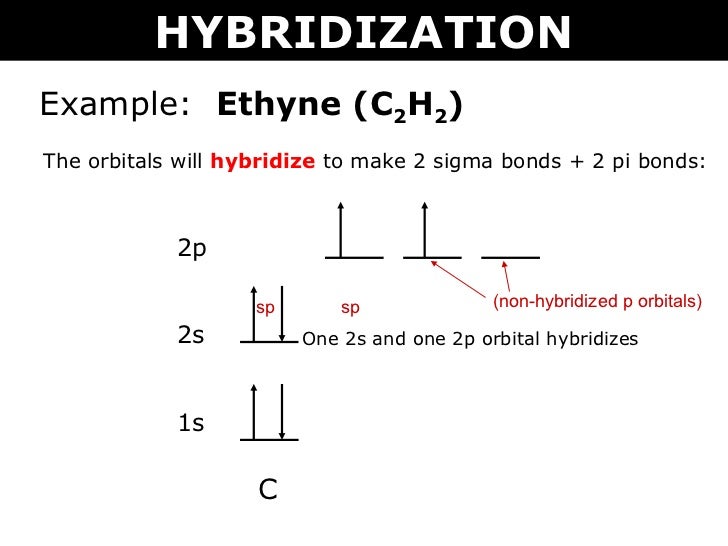
In the formation of C2H2, the carbon atom needs extra electrons to form 4 bonds with hydrogen and other carbon atoms. As a result, one 2s 2 pair is moved to the empty 2pz orbital. The 2s orbital in each carbon hybridizes with one of the 2p orbitals and forms two sp hybrid orbitals. Ethyne has a triple bond between the two carbon atoms.
C2H2 (acetylene or ethyne) contains two carbon atoms and two hydrogen atoms. There is a triple bond between carbon atoms. There are no lone pairs on carbon or hydrogen atoms. In this tutorial, we are going to learn how to draw lewis structure of C2H2.
Is C2H2 linear or bent? C2H2 has a linear shape given its molecular geometry is linear and all the atoms are arranged symmetrically. To summarize this article on C2H2 Lewis structure, we can say that, There are ten valence electrons for Ethyne.
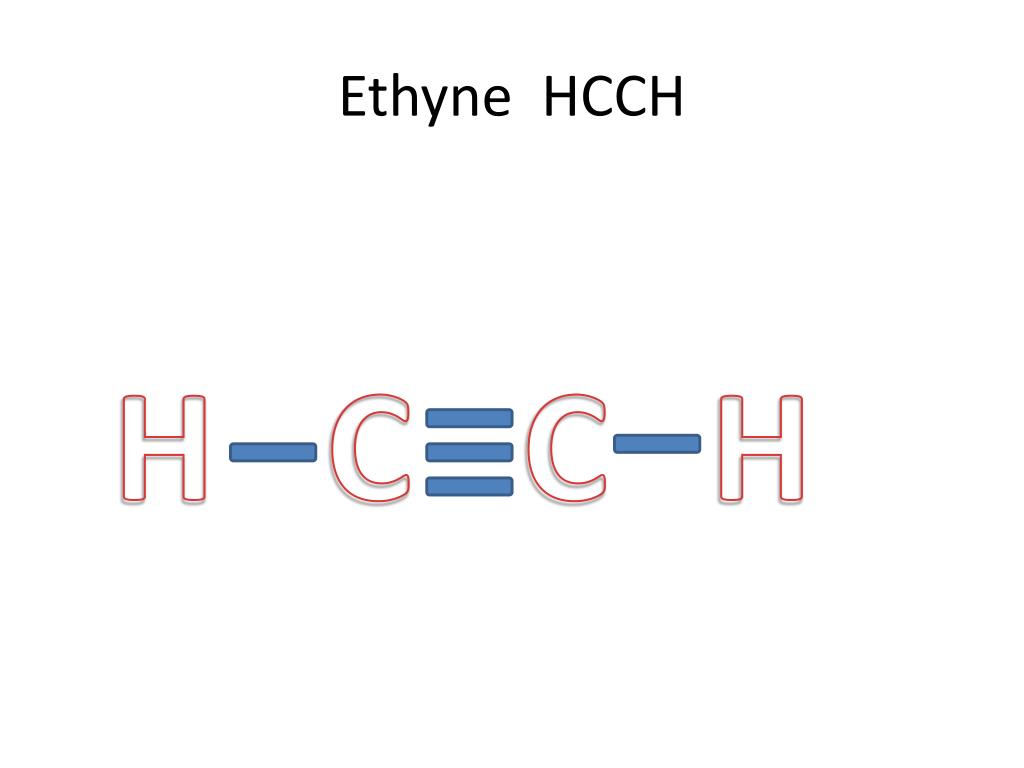
Hcch lewis structure.HCCH is also known as C2H2 Ethyne and Acetylene For the HCCH Lewis structur. Ethyene ethylene CH 2 CH 2. CH 3 CH 2 OH. The carbons are linear so they are sp. B Using valence bond theory draw an energy diagram of the orbitals in acetylene similar to the one we drew for ethylene in class.
The lewis structure of c2h2 has 1 triple bond. For the HCCH Lewis structure youll need to form a triple bond between the two carbon atoms. Triple bonds are always shorter than single bonds. There are no lone pairs on carbon or hydrogen atoms. For each C one can explain the bonds through sp hybridization a triple bond and one single bond.
I quickly take you through how to draw the Lewis Structure of CHCH (Acetylene or ethyne). I also go over hybridization, shape, sigma, pi bonding and bond ang...
Hey Guys,In this video we are going to learn about the Lewis structure of C2H2. It is a chemical formula for Ethyne or Acetylene.To understand the Lewis stru...
C2h2 Acetylene Ethyne Lewis Structure . C2h2 Ve C2h6 Nin Lewis Gosterimi Acil Yapabilir Misiniz Eodev Com . How To Determine The Lewis Dot Structure For C2h2cl2 Quora . Http Www Youtube Com Watch V Qojoaosk5ui Lewis Chemistry Math Equations . Location: Share : Newer Posts Older Posts
on Lewis Dot Diagram For C2h2. 70 More Lewis Dot Structures. Since all the atoms are in either period 1 or 2, this molecule will adhere to the octet rule. The exception, of course, being the. This is a total of 10 valence electrons that have to be included in the Lewis structure. The three lines between the carbon atoms represent 8 valence ...
A step-by-step explanation of how to draw the C2H4 Lewis Dot Structure (Ethene).For the C2H4 structure use the periodic table to find the total number of val...
A quick explanation of the molecular geometry of C2H2 including a description of the C2H2 bond angles. Note, the Hydrogen atoms (H) should not have lone pair...
Get the free "Lewis structure" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.























0 Response to "41 lewis diagram for c2h2"
Post a Comment