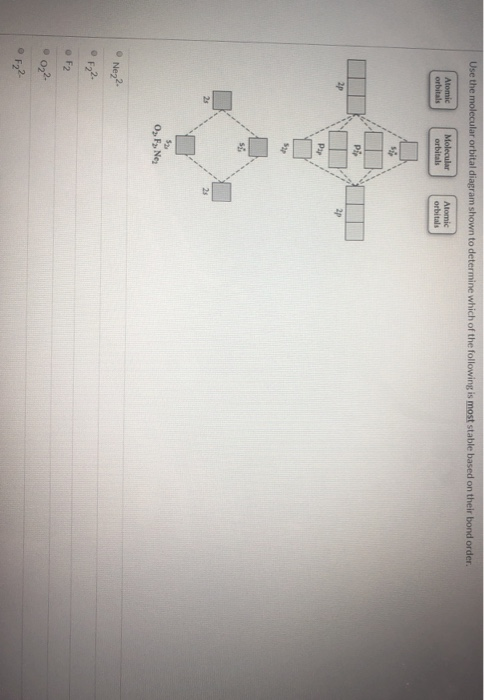
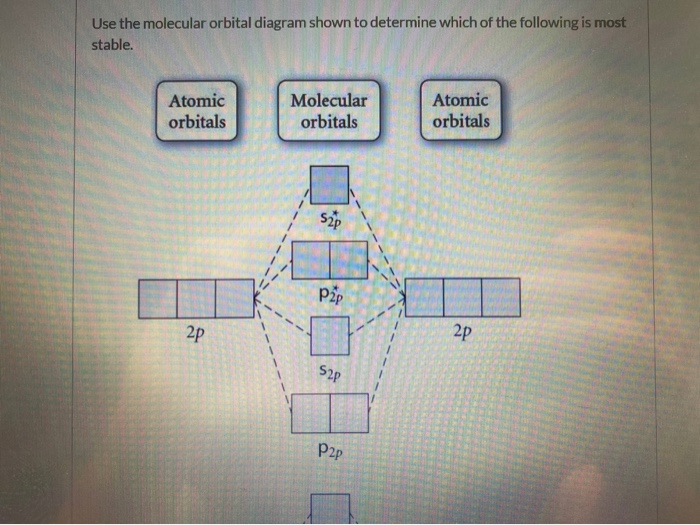
37 draw the molecular orbital diagram shown to determine which of the following is most stable.
Draw the molecular orbital diagram shown to determine which of the following is most stable. Draw the molecular orbital diagram shown to determine which of the following is most stable. F22+ F2 Ne22+ O22+ F22-. Question: Draw the molecular orbital diagram shown to determine which of the following is most stable. Draw the molecular orbital diagram shown to determine which of the following is most stable. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. C22- should have the highest bond order (3, it has 6 more e ….
Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. F₂²⁺ Draw the molecular orbital diagram shown to determine which of the following is most stable.
Draw the molecular orbital diagram shown to determine which of the following is most stable.
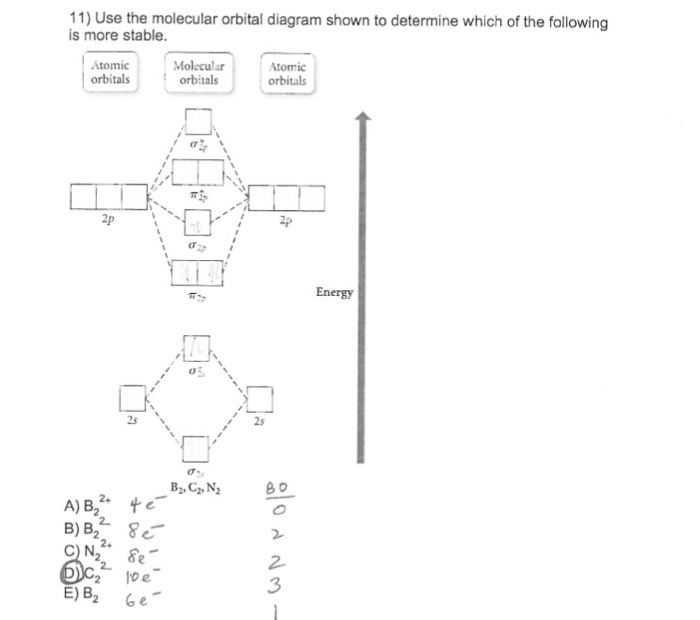
Draw the molecular orbital diagram shown to determine which of the following is most stable. Draw the molecular orbital diagram shown to determine which of the following is most stable. F22- O22+ F2; Question: Draw the molecular orbital diagram shown to determine which of the following is most stable. Draw the molecular orbital diagram shown to ... 31) Use the molecular orbital diagram shown to determine which of the following is most stable. A) C2^2⁺ B) N2^2⁺ C) B2; D) C2^2⁻ E) B2^2⁺ 32) How many moles of oxygen are formed when 58.6 g of KNO3 decomposes according to the following reaction? The molar mass of KNO3 is 101.11 g/mol. 4 KNO3(s) → 2 K2O(s) + 2 N2(g) + 5 O2(g) Construct the molecular orbital diagram for each molecule and determine which is most stable by counting the electrons in anti-bonding MOs.. Recall that as the electrons in the anti-bonding MO (denoted by *) increases, the more unstable the compound is.. For this matter, we have to look for the molecule with the least amount of electrons in the antibonding MO
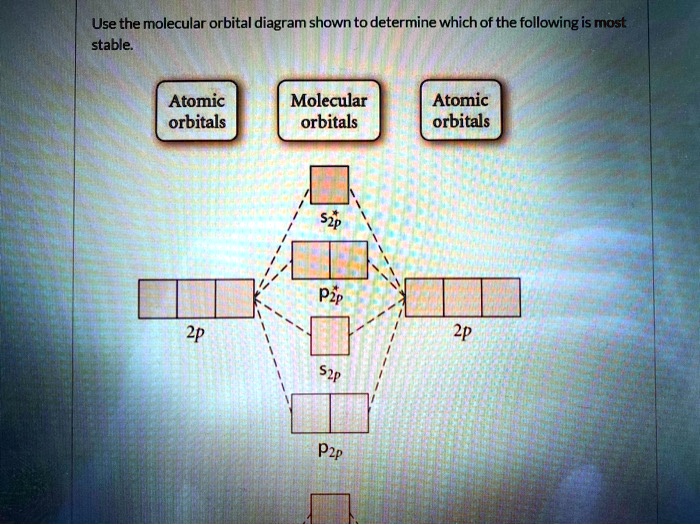
Draw the molecular orbital diagram shown to determine which of the following is most stable.. Draw the molecular orbital diagram shown to | Chegg.com. 34. Draw the molecular orbital diagram shown to determine which of the following is most stable a. C22* b. N22 c. B2 d. C22 e. B22. Question: 34. Draw the molecular orbital diagram shown to determine which of the following is most stable a. Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons Draw the molecular orbital diagram for the oxygen molecule, O 2. From this diagram, calculate the bond order for O 2. How does this diagram account for the paramagnetism of O 2? Solution We draw a molecular orbital energy diagram similar to that shown in . Diff: 4 Page Ref: 10.8 58) Draw the molecular orbital diagram shown to determine which of the following is most stable. A) F 2 B) F 2 2 ⁺ C) Ne 2 2 ⁺ D) O 2 2 ⁺ E) F 2 2 ⁻ Answer: D Diff: 5 Page Ref: 10.8 Draw the Molecular orbital Diagram Shown to Determine which Of the Following is Most Stable. use the molecular orbital diagram shown to determine which use the molecular orbital diagram shown to determine which of the following is most stable 38 a b2 b c22 use the molecular orbital question draw the molecular orbital diagram shown to answer to draw the molecular orbital diagram shown to ...
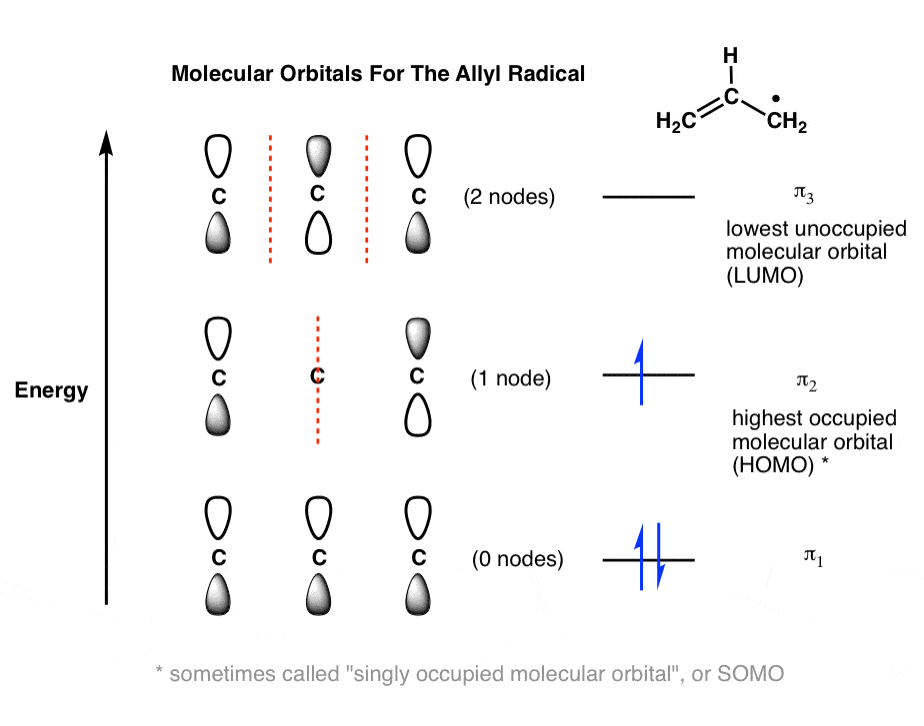
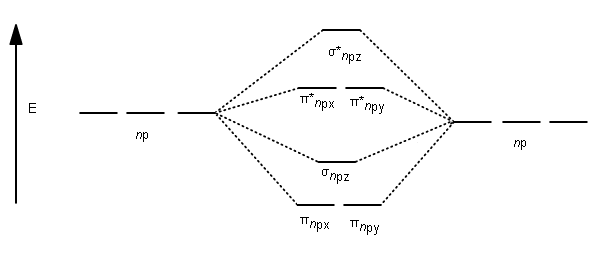
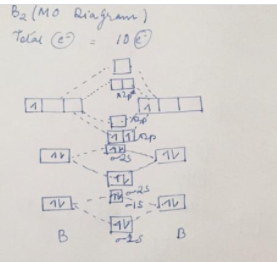
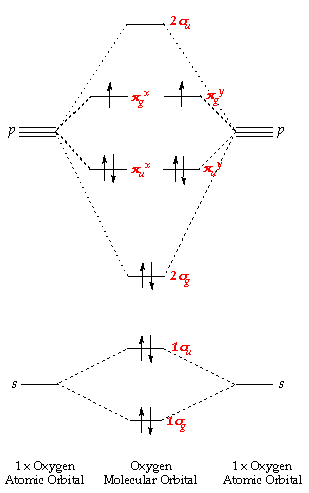
Draw the molecular orbital diagram shown to determine which of the following is most stable. A) F2 B) F22⁺ C) Ne22⁺ D) O22⁺ E) F22⁻ D) O22⁺ Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. A) O22⁻ ... Draw the molecular orbital diagram shown to determine which of the following is most stable. F22-. Ne22+. O22+. F22+. F2. Best Answer. This is the best answer based on feedback and ratings. 100% (12 ratings) more stable than on the individual atoms, this is referred to as a bonding molecular orbital. A second molecular orbital is also created, which we simplistically show as a subtraction of the two atomic 1s orbitals [σ* = (1sa - 1sb)]. This orbital is called sigma-star (σ*) and is less stable than the two separated atoms. Because it is less ... The molecular orbital diagrams for molecules and ions are drawn from the order of increasing energies shown in the molecular orbital configuration. Always remember that the number of molecular orbitals formed must be equal to the number of atomic orbitals that were combined in the molecule.
Apr 28, 2017 — Use the molecular orbital diagram shown to determine which of the following is most stable. A. F22+ B. Ne2… Get the answers you need, now!2 answers · Top answer: Answer:The most stable element based on the molecular orbital diagram is [tex]\text ... Give the electron configurations for the species C2 and C When we draw the C2 MO, we have everything up till the PiPy Orbitlal filled, and the next orbital tht would be filled would be the sigma2Pz. Answer to Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. N22+ B22+ B B2 CeV. FREE Answer to Use the molecular orbital diagram shown to determine which of the following is most stable. A....1 answer · Top answer: Concepts and reason Bond order of the molecule indicates the number of bond present between the pair of atoms. Bond order is the measurement of ... Draw the molecular orbital diagram shown to determine which of the following is most stable. O22, C22- Choose the compound below that contains at least one polar covalent bond, but is nonpolar.
Draw the molecular orbital diagram shown to determine which of the following is most stable. O22+ Identify the number of electron groups around a molecule with a : tetrahedral shape
2. The bond order of a homonuclear diatomic molecule can be decreased by. removing electrons from a bonding MO or adding electrons to an antibonding MO. Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. A) N2^2+. B) B2^2+. C) B2^2-. D) C2^2-. E) B2.
We're being asked which species is the most stable. For this, we need to determine the bond order for each species. The bond order tells us the stability of a bond: a higher bond order means the bond is more stable. Step 1: Calculate the total number of valence electrons present. Step 2: Draw the molecular orbital diagram.
Problem: Use the molecular orbital diagram shown to determine which of the following is most stable.a.F22+b. Ne22+c. F22-d. O22+e. F2
Use molecular orbital diagram shown to determine which is most stable a) O22-b)F2 c) F22+ d) F22-e) Ne22-a. Use the molecular orbital diagram shown to determine which of the following is most stable. A) F2 B) F22⁺ ... Draw the lewis structure for C3H6. How many sigma and pi bonds? a) 8,2 b) 9,1 c) 8,1 d) 9,0
3) Draw the molecular orbital diagram needed, and determine which of the following is paramagnetic. A) B2^2+ B) B2^2-C) N2^2+ D) C2^2-E) B2; 4) Draw the molecular orbital diagram shown to determine which of the following is most stable. A) C2^2+ B) N2^2+ C) B2; D) C2^2-E) B2^2+ 5) Which statement regarding stable heteronuclear diatomic ...
Construct the molecular orbital diagram for each molecule and determine which is most stable by counting the electrons in anti-bonding MOs.. Recall that as the electrons in the anti-bonding MO (denoted by *) increases, the more unstable the compound is.. For this matter, we have to look for the molecule with the least amount of electrons in the antibonding MO
31) Use the molecular orbital diagram shown to determine which of the following is most stable. A) C2^2⁺ B) N2^2⁺ C) B2; D) C2^2⁻ E) B2^2⁺ 32) How many moles of oxygen are formed when 58.6 g of KNO3 decomposes according to the following reaction? The molar mass of KNO3 is 101.11 g/mol. 4 KNO3(s) → 2 K2O(s) + 2 N2(g) + 5 O2(g)
Draw the molecular orbital diagram shown to determine which of the following is most stable. Draw the molecular orbital diagram shown to determine which of the following is most stable. F22- O22+ F2; Question: Draw the molecular orbital diagram shown to determine which of the following is most stable. Draw the molecular orbital diagram shown to ...
























0 Response to "37 draw the molecular orbital diagram shown to determine which of the following is most stable."
Post a Comment