39 match the appropriate octahedral crystal-field splitting diagram fe4+
Fig.9.8: d orbital splitting in an octahedral crystal field The crystal field splitting, Δ↓o, depends upon the field produced by the ligand and charge on the metal ion. Some ligands are able to produce strong fields in which case, the splitting will be large whereas others produce weak fields and consequently result in small splitting of d ... Chemistry questions and answers. Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Metal ion and spin state Octahedral splitting diagram IIIIIIIIIIIIIIIIIIIIIIIII Answer Bank high-spin Fe4+ low-spin Mn3+ 11 1 1 1 11 1 1.
Academia.edu is a platform for academics to share research papers.
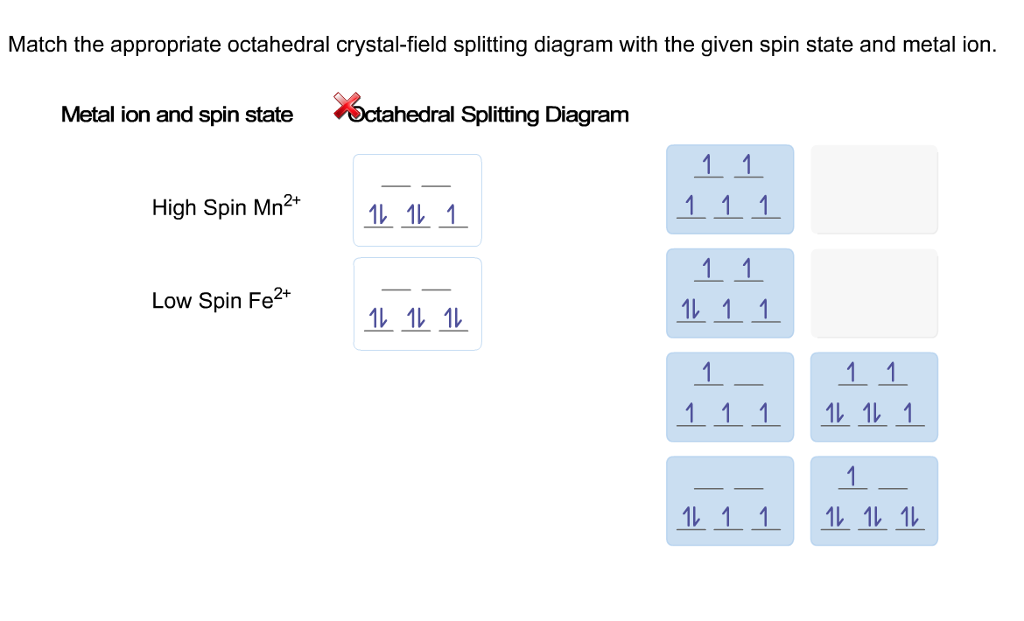
Match the appropriate octahedral crystal-field splitting diagram fe4+
Orgel Diagrams 26.16 Racah Parameters 26.24 Terms Correlation Diagrams under the Effect of Weak and Strong Field Effects 26.26 Tanabe-sugano Diagrams (T-S Diagram) 26.29 Charge-Transfer Transitions 26.34 Types of Magnetism 26.39 Summary 26.55 Solved Examples 26.56 Exercises 26.57 27. Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. high spin Ni 4+ low spin Fe 3+ ...Missing: fe4 | Must include: fe4 Question: Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. High Spin Fe^4+ Low Spin Mn^3+ ...Missing: fe4 | Must include: fe4
Match the appropriate octahedral crystal-field splitting diagram fe4+. Metal ion and spin state Octahedral Splitting Diagram 1L 11 1 High Spin Fe2+ Low Spin Ni3 1L 11 1L 111. This problem has been solved! See the answer ...Missing: fe4 | Must include: fe4 Solid State ChemiStry and itS appliCation 2014 Anthony R. West (b) Crystal field splitting in tetrahedral coordination entities : In tetrahedral coordination entity formation, the d orbital splitting is inverted and is smaller as compared to the octahedral field splitting. For the same metal, the same ligands and metal-ligand distances, it can be shown that t = (4/9) 0. This may attributes to the following ... Given below are some important topics of Physical, Organic and Inorganic that require special attention: Physical: ∑ Bohr's theory of atomic structure, quantum numbers and orbitals. ∑ MO approach to diatomic molecules, concepts of hybridization/VSEPR theory. ∑ Van der Waals equation of state and its application to the behaviour of real ...
Question: Match the appropriate octahedral crystal-field splitting diagram with for the metal ion with the given spin state.Missing: fe4 | Must include: fe4 06.04.2014 · Transcribed image text: Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion Metal ion and spin state Octahedral Splitting Diag High Spin Mn? 1 1 Low Spin Co2 1L 1L 1L 1L 1L 1L 1 1 1 1 1 Question- Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. High spin Cr^2+ Low spin Fe^3+ Please give the octahedral splitting diagrams for each ion. We find that the square planar complexes have the greatest crystal field splitting energy compared to all the other complexes. Match the appropriate ...
Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Metal ion and spin state Octahedral Splitting Diagram High Spin Ni3+ Low Spin Fe 4+ Question: Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Metal ion and spin state Octahedral ... Match the appropriate octahedral crystal field splitting diagram fe4. Question match the appropriate octahedral crystal field splitting diagram with the given spin state and metal ion. Construct the octahedral crystal field splitting diagram for the metal in each species. High spin mn2 low spin co3 this problem has been solved. W9.1 Jahn-Teller Effect 75 W9.2 Examples of Weak and Strong Crystal Field Effects 75 W9.3 Crystal Fields and Cr3C in Al2 O3 75 W9.4 Experimental Results for in the Free-Spin Limit 78 W9.5 Spin Glasses and the RKKY Interaction 79 W9.6 Kondo Effect and s-d Interaction 79 W9.7 T for Ni 80 xii WEB CONTENTS 29.03.2019 · Question match the appropriate octahedral crystal field splitting diagram with the given spin state and metal ion. Perovskite And Related Structure Systems Crystal field splitting in an octahedral field eg energy 35 o o 25 o t2g e g the higher energy set of orbitals d z2 and d x2 y2 t 2g the lower energy set of orbitals d xy d yz and d xz δ o or 10 dq the energy …
No of octahedral voids E. 4r =3a defects present in fcc unit cell MATCH THE FOLLOWING MATCH THE FOLLOWING-5 MATCH THE FOLLOWING-6 COLUMN -I COLUMN -II COLUMN -I COLUMN-II 1.The process of adding an A.Ferrimagnetism 1.Paramagnetism A.Intrinsic appropriate amount of semiconductor suitable impurity to increase the conductivity of semiconductor .
Crystal Field Splitting in an Octahedral Field eg Energy 3/5 o o 2/5 o t2g e g - The higher energy set of orbitals (d z2 and d x2-y2) t 2g - The lower energy set of orbitals (d xy, d yz and d xz) Δ o or 10 Dq - The energy separation between the two levels The eThe eg orbitals are repelled by an amount of 0 6orbitals are repelled by an amount ...
Unit 3 Electrochemistry 63 3.1 Electrochemical Cells 64 3.2 Galvanic Cells 65 3.3 Nernst Equation 68 3.4 Conductance of Electrolytic Solutions 73 3.5 Electrolytic Cells and Electrolysis 83 3.6 ...
24 THE CRYSTAL FIELD 13 Other Geometries In 4 coordination, two geometries are common, tetrahedral and square planar, for which the crystal eld splitting patterns are shown in Fig. 1.4. For the same ligand set, the tetrahedral splitting parameter is smaller than that for the octahedral geometry by a factor of 23 because we now have only four ...
Chemistry questions and answers. Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Metal ion and spin state Octahedral splitting diagram Answer Bank high-spin Mn3+ low-spin Fe4+ 2 12.
Metal ion and spin state Octahedral Splitting DiagramHigh Spin Mn2Low Spin Fe4 Match the appropriate octahedral crystal-field splitting diagram with the ...
Crystal-electric field splitting and the symmetry of 3d orbitals (a) CEF splitting at tetrahedral and octahedral lattice sites. Due to the lattice symmetry and shape of the orbitals, the splitting at the octahedral sites is significantly larger. In tetrahedral symmetry the ground state is labelled e.
Ans. If the element present in high spin …. View the full answer. Transcribed image text: Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Metal ion and spin state Octahedral splitting diagram Answer Bank high-spin Fe4+ 1 1 1 1 1 1 1 1 low-spin Co2+ 1 1 1 1 1 1 11 1 1 1 1 1 1 1 1 1 1.
Academia.edu is a platform for academics to share research papers.
Match the appropriate octahedral crystal-field splitting diagram with the ... ion and spin state Octahedral splitting diagram Answer Bank high-spin Fe4 + ...4 answers · Top answer: Doctor Octopus Federal crystal splitting field. Chromium three. Plus Looks like that for ...
Chemistry. Chemistry questions and answers. Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Metal ion and spin state Octahedral splitting diagram Answer Bank high-spin Ni3+ low-spin Fe4+. Question: Match the appropriate octahedral crystal-field splitting diagram with the given spin state ...
Academia.edu is a platform for academics to share research papers.
01.12.2019 · A d1 octahedral complex is found to absorb visible light, with the absorption maximum occcurring at nm. a) Calculate the crystal-field splitting energy, Δ, in. Solved: Construct the octahedral crystal-field splitting diagram for the metal in each species. a) [math]V(H_2O)_6^{3+}[/math] a) [math]Co(CN)_6^{3-}[/math] a) .(The energy gap,, is ...
S denotes the ground-state spin and J is the (weak-crystal-field) effective total angular momentum into which the substitutional ground-state term is split by spin-orbit interaction in first order. The J > 3/2 of ' E is further split in second order.
Chemistry questions and answers. Match the appropriate octahedral crystal-field splitting diagram with for the metal ion with the given spin state. Metal ion and spin state Octahedral splitting diagram Answer Bank High-spin Co3+ 11 Low-spin Co3+ 1 1 1 1 1L 1 1 1.
Let us explain this splitting in different crystal fields. (a) Crystal field splitting in octahedral coordination entities In an octahedral coordination entity with six ligands surrounding the metal atom/ion, there will be repulsion between the electrons in metal d orbitals and the electrons (or negative charges) of the ligands.
Thus the size of the octahedral gaps differs in the different structures and therefore the crystal fields induced by a the oxygen dianions too. 2-By means of the shorter distance O - metal in ruby, the octahedral crystal field of ruby is stronger than that of chromium oxide. This leads to a larger splitting of the t2g eglevels in ruby.
Question: Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. High Spin Fe^4+ Low Spin Mn^3+ ...Missing: fe4 | Must include: fe4
Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. high spin Ni 4+ low spin Fe 3+ ...Missing: fe4 | Must include: fe4
Orgel Diagrams 26.16 Racah Parameters 26.24 Terms Correlation Diagrams under the Effect of Weak and Strong Field Effects 26.26 Tanabe-sugano Diagrams (T-S Diagram) 26.29 Charge-Transfer Transitions 26.34 Types of Magnetism 26.39 Summary 26.55 Solved Examples 26.56 Exercises 26.57 27.
0 Response to "39 match the appropriate octahedral crystal-field splitting diagram fe4+"
Post a Comment