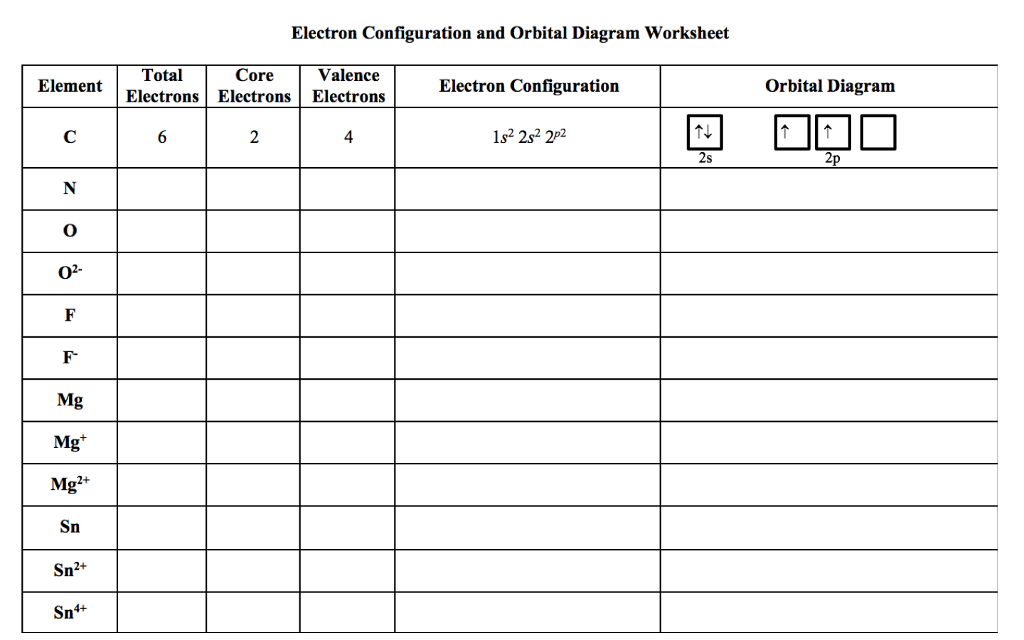
40 electron configuration and orbital diagram worksheet
This worksheet provides extra practice for writing electron configurations. Use helium and neon as examples to illustrate your explanation. Electron configuration worksheet w 311 everett community college tutoring center student support services program write the unabbreviated electron configurations of the following elements. Write the complete. calcium has 20 electrons. No matter what the atom is, the orbital structure is the same. In class we will learn how to use the periodic table to remember the orbital structure, and then write it using the shorthand notation of electron configurations. Some things to remember: • Each orbital can contain 0, 1, or 2 electrons (and no more!).
HW #6 - Electron Configuration and Orbital Diagrams Directions: Please fill in the electron configuration for the following elements and then draw the orbital diagram configuration in the appropriate boxes. F Electron Configuration Mg Electron Configuration Orbital Diagram Configuration Orbital Diagram Configuration B Electron Configuration He

Electron configuration and orbital diagram worksheet
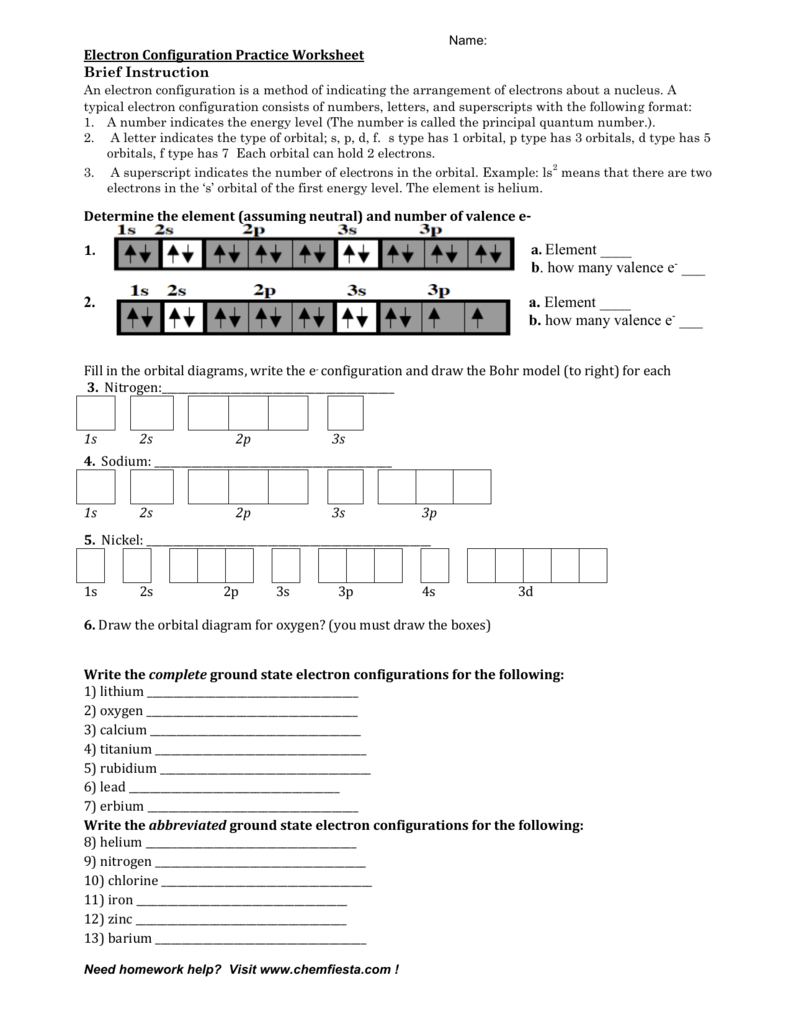
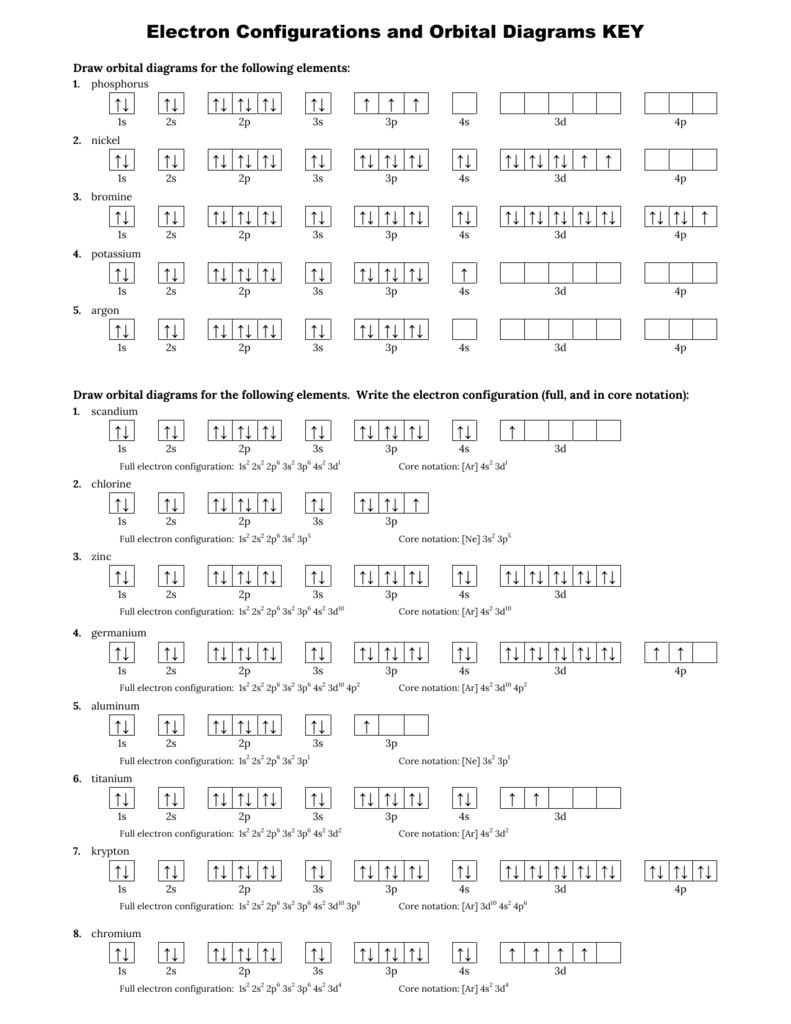
Chemistry Worksheet - Review for Electronic Structure of Atoms Page 3. Write the electron configuration (ie: 1s2, 2s1), orbital diagram, noble gas notation, and Lewis dot notation for: H, Li, Na, and K atoms. 2. Be, Mg and Ca atoms. 3. C, Si and Ge atoms. 4. F, Cl and Br atoms. 5. Fe, Ni and Zn atoms. 6. S -2 and K1+ ions. 7. Mg2+ and F1- ions Electron Configurations - Solutions Note: The electron configurations in this worksheet assume that lanthanum (La) is the first element in the 4f block and that actinium (Ac) is the first element in the 5f block. If your periodic table doesn't agree with this, your answers for elements near the f-orbitals may be slightly different. An electron configuration is a method of indicating the arrangement of electrons about a nucleus. A typical electron configuration consists of numbers, letters, and superscripts with the following format: 1. A number indicates the energy level (The number is called the principal quantum number.). 2. A letter indicates the type of orbital; s, p, d, f.
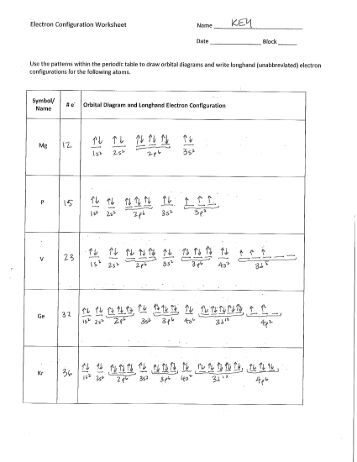
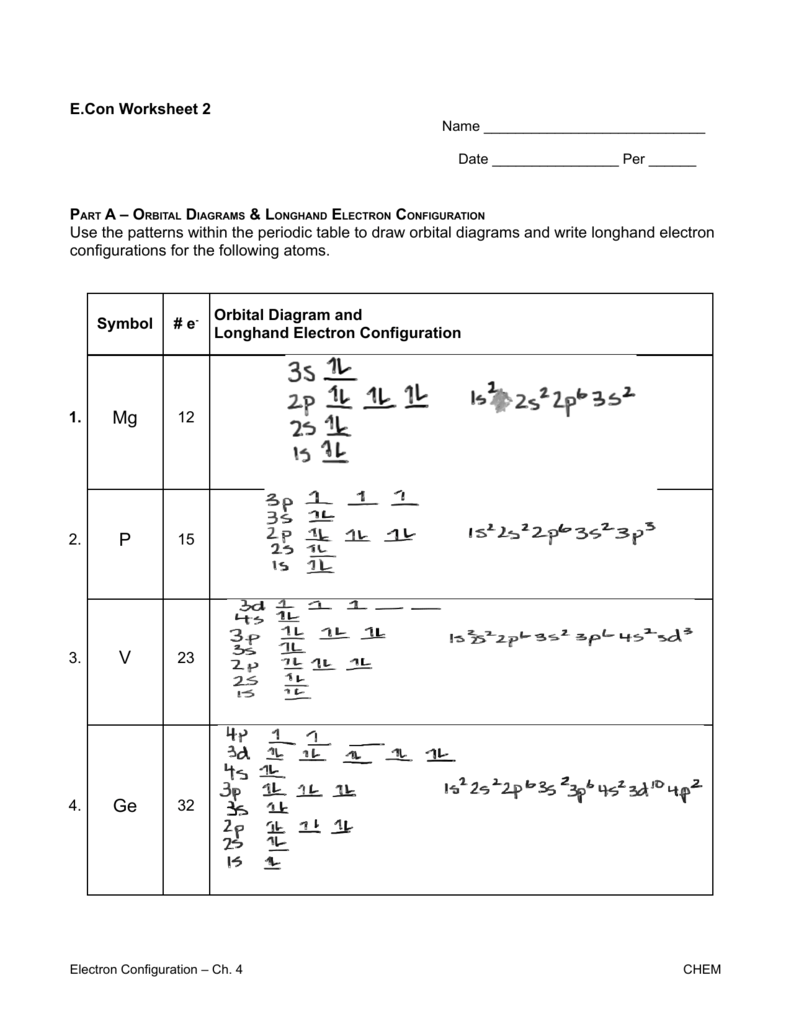
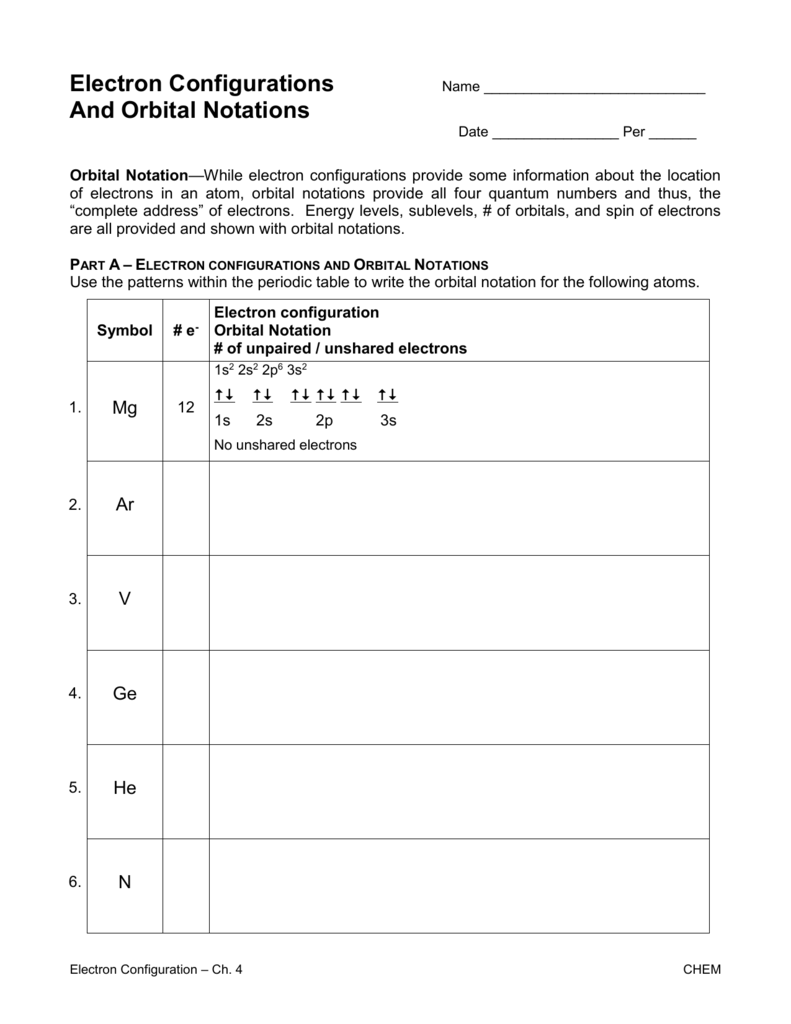
Electron configuration and orbital diagram worksheet. Use the patterns within the periodic table to draw orbital diagrams and write longhand electron configurations for the following atoms. Symbol # e- Orbital Diagram and Longhand Electron Configuration 1. Mg . 1s 2s 2p 3s 3p 4s 3d Electron configuration: 2. P . 1s 2s 2p 3s 3p 4s 3d Electron configuration: 3. V . 1. Fill in the electron configurations for the elements given in the table. Use the orbital filling diagrams to complete the table. Is 2s lectron Is 4s on 2s a o o gurations or ome Orbital filling elected ements Electron 3s configuration Isl C] element (answer) en on Element O Ne 2Px 2py 2pz 2. Which element has the following orbital diagram? 3. There are lots of quizzes on electron configurations you can practice with located here. Orbital Diagrams. Another way to represent the order of fill for an atom is by using an orbital diagram often referred to as "the little boxes": The boxes are used to represent the orbitals and to show the electrons placed in them. Electron Configurations Worksheet Write the complete ground state electron configurations and orbital notations for the following: # of e - Element (atom) e - configuration Orbital Notations/ diagrams
Name _____per_____ Orbital Diagrams and Electron Configuration Worksheet: Part A: Using the Periodic Table, first give the number of protons and electrons in the neutral atom of the given element. On the line above the brackets, write the electron configuration for each element. Actual Electron Configurations •Total electrons = atomic number •Fill energy levels with electrons until you run out •A superscript states how many electrons are in each level –Hydrogen – 1s1 – 1 electron total –Helium – 1s2 – 2 electrons total –Lithium – 1s22s1 – 3 electrons total –Beryllium – 1s22s2 – 4 electrons total Purpose: Electron orbital diagrams are a way of illustrating what energy level and orbital shape of the probable location of each of the electrons of an element.In this worksheet, students will learn how to properly fill in the arrows of electron orbital diagrams, following Hund's Rule, the Pauli Exclusion Principle, and the Aufbau Principle. 9. Fill in the electron configurations for the elements given in the table. Use the orbital filling diagrams to complete the table. 2 Electron Configurations for Some Selected Elements Orbital filling Element 1s 2s 2px 2py 2pz 3s Electron configuration He C O Ne 1s1 1s22s1 1s2s22p3 1s22s22p5 1s22s22p63s1
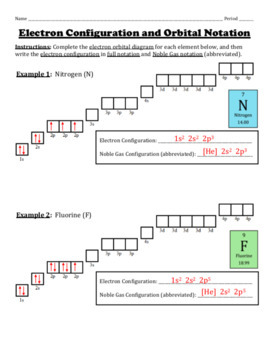
Write the full electron configuration, short-hand electron configuration, and fill in the orbital diagrams, for the following elements. 1. Nitrogen IS 35 2. Chlorine Is 25 3. Sodium Is 2s 35 I 4. Neon 3p 3p 3p IS 25 . Electron Configuration Practice Chemistry 5. Nickel Name : Practice 1: Electron Configurations. For each of the following write the complete electron configuration: Ex. Al =1s22s22p63s23p1. O - 1s22s22p4. Mg - 1s22s22p63s2. Ar - 1s22s22p63s23p6. K - 1s22s22p63s23p64s1. Zn - 1s22s22p63s23p64s23d10. ... Orbital Notation Worksheet ... This great review worksheet requires students to write the electron configurations and draw the orbital diagrams for four elements. Students then find the element for seven different electron configurations and learn how to do noble gas shorthand. Electron Configurations, Orbital Notation and Quantum Numbers 318 Laying the Foundation in Chemistry 5 • Transition metals generally have an oxidation state of +2 since they lose the s2 that was filled just before the d-sublevel began filling.
About the Electron Configuration Worksheet Finally, the easy way to learn how to find an electron configuration, also known as an orbital diagram or the quantum numbers. Download the Naming Electron Configuration Worksheet Download and print the black and white pdf. It's 5 printer-friendly pages. There's an answer key too in the other pdf file.
Electron configuration and orbital diagram worksheet pdf The purpose of introducing quantum numbers is to show that the similarity of electron arrays or electron compositions leads to similarities and differences in the characteristics of elements.
The electron configurations in this worksheet assume that lanthanum la is the first element in the 4f block and that actinium ac is the first element in the 5f block. Electron configuration and orbital diagram worksheet diagram for. The further the electron is from the nucleus the _____ energy the electron has.
Electron Configuration and Orbital Diagram Worksheets - On these two practice worksheets, students write full electron configurations and noble gas configurations, complete orbital diagrams, and mark valence electrons. Worksheet #1 (2 pages) - Includes notes and a walkthrough example on solving el
Electron Orbitals: Electron Configuration Orbital Diagram Worksheet Answers (The electron configuration orbital diagram worksheet answers can be found at the bottom of the lesson.) The 2, 8, 8, 18 rule is a very simplistic view of electron configuration and doesn't give the full picture when it comes to electron configuration.
Reminder of electron configuration rules: Aufbau Principle: Electrons occupy lowest energy levels first. Pauli Exculsion principle: Each orbital contains up to 2 electrons. If an orbital contains 2 electrons, they must have opposite “spin.” Hund’s rule: One electron must occupy each orbital in a sublevel before a second electron occupies an orbital. Part B – Rules of Electron Configurations
Chemistry. Chemistry questions and answers. Electron Configurations Worksheet Write the complete ground state electron configurations and orbital notations for the following: #of e Element (atom) e configuration Orbital Notations/ diagrams 1) 3 lithium 15^2 2541 [1L] [1] 2) 8 oxygen 18^2 25^2 2P44 [1L] [1L] [11] [1] [1] [11] [11] [14] [14] [14 ...
Electron Configurations and Periodic Trends 1. Rt has three extra electrons 11 02 2 12. Orbital Diagrams Doc Chemistry Classroom Teaching Chemistry Electron Configuration Just fancy it by voting.Electron configuration worksheet 3 answer key. The electron configurations in this worksheet assume that lanthanum la is the first element in the 4f block and that actinium […]
This worksheet provides extra practice for writing electron configurations. The easiest and most reliable technique for writing electron configurations is to use the periodic table as your guide. Details of using the periodic table as a guide for determining electron configurations can be found on the CH301 website. 1.
Worksheet 4 - Electron Configurations. Worksheet 5 - Orbital and Energy Level Diagrams. Worksheet 6 - Quantum Numbers ... electron configurations for each of the following atoms. phosphorus. 1s. 2. 2s. 2. 2p. 6. 3s. 2. 3p. 3 [Ne]3s. 2. 3p. 3. ... Identify and correct the errors in each of the following valence shell orbital diagrams ...
An electron configuration is a method of indicating the arrangement of electrons about a nucleus. A typical electron configuration consists of numbers, letters, and superscripts with the following format: 1. A number indicates the energy level (The number is called the principal quantum number.). 2. A letter indicates the type of orbital; s, p, d, f.
Electron Configurations - Solutions Note: The electron configurations in this worksheet assume that lanthanum (La) is the first element in the 4f block and that actinium (Ac) is the first element in the 5f block. If your periodic table doesn't agree with this, your answers for elements near the f-orbitals may be slightly different.
Chemistry Worksheet - Review for Electronic Structure of Atoms Page 3. Write the electron configuration (ie: 1s2, 2s1), orbital diagram, noble gas notation, and Lewis dot notation for: H, Li, Na, and K atoms. 2. Be, Mg and Ca atoms. 3. C, Si and Ge atoms. 4. F, Cl and Br atoms. 5. Fe, Ni and Zn atoms. 6. S -2 and K1+ ions. 7. Mg2+ and F1- ions













![[DIAGRAM] Electron Configuration And Orbital Diagram ...](https://i.ytimg.com/vi/zsEqOYKistM/maxresdefault.jpg)








0 Response to "40 electron configuration and orbital diagram worksheet"
Post a Comment