Answer to 25 Free Energy (kcal/mol) 20 15 10 B A C D O Reaction progress Use the reaction energy diagram above to answer the following questions.1 answer · Top answer: See explanation. DeltaG=Gproduct-Greactant Use the reaction energy diagram above to answer the following questions. Calculate the activation energy, ?G, for the step D to C. ______ kcal/mol.1 answer · Top answer: Faster...
Use the reaction energy diagram above to answer the following questions. Calculate the activation energy, ΔG, for the step C to D. _____ kcal/mol Calculate ...4 answers · Top answer: Look at problems 76. So one to determine is this excellent and a thermic. So for example, ...
Use the reaction energy diagram above to answer the following questions.
FREE Answer to Free Energy (kcal/mol) Reaction progress- Use the reaction energy diagram above to answer the following questions....1 answer · 0 votes: FOr the D to c = 12-7=5K Cal/mok to A 7-12--5 KCallmal FOr B toA A S 18-12 =6 K cadl mol c toD 4# ster c toD iS tast er o0ttom Higure 12-8 =4K Cal/mol ... Problem: Use the reaction energy diagram above to answer the following questions. Calculate the activation energy, ΔG for the step C to B _____ kcal/mol ...3 Dec 20201 answer · Top answer: Activation energy → Highest peak - Starting EnergyFor step B to C, step is at 12 kcal/mol while the peak is at 24 kcal/mol24 - 12 = 12 kcal/mol[readmore]There ...
Use the reaction energy diagram above to answer the following questions.. Problem: Use the reaction energy diagram above to answer the following questions. Calculate the activation energy, ΔG for the step C to B _____ kcal/mol ...3 Dec 20201 answer · Top answer: Activation energy → Highest peak - Starting EnergyFor step B to C, step is at 12 kcal/mol while the peak is at 24 kcal/mol24 - 12 = 12 kcal/mol[readmore]There ... FREE Answer to Free Energy (kcal/mol) Reaction progress- Use the reaction energy diagram above to answer the following questions....1 answer · 0 votes: FOr the D to c = 12-7=5K Cal/mok to A 7-12--5 KCallmal FOr B toA A S 18-12 =6 K cadl mol c toD 4# ster c toD iS tast er o0ttom Higure 12-8 =4K Cal/mol ...

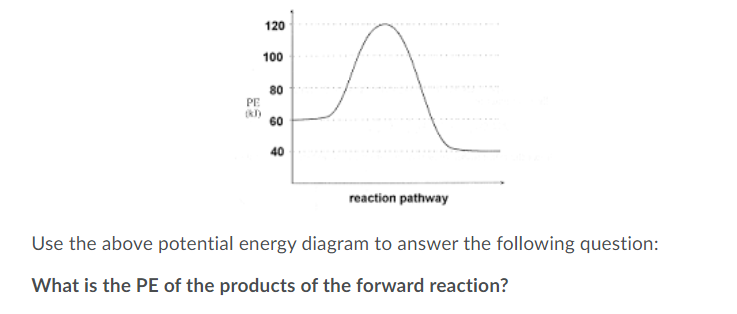
Answered: 120 100 80 PE k) .... 60 40 reaction… | bartleby
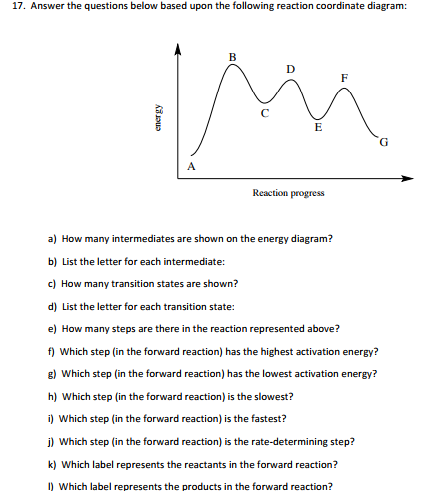
Solved 17. Answer the questions below based upon the | Chegg.com

Consider the reaction: A + B → C. The diag... | Clutch Prep
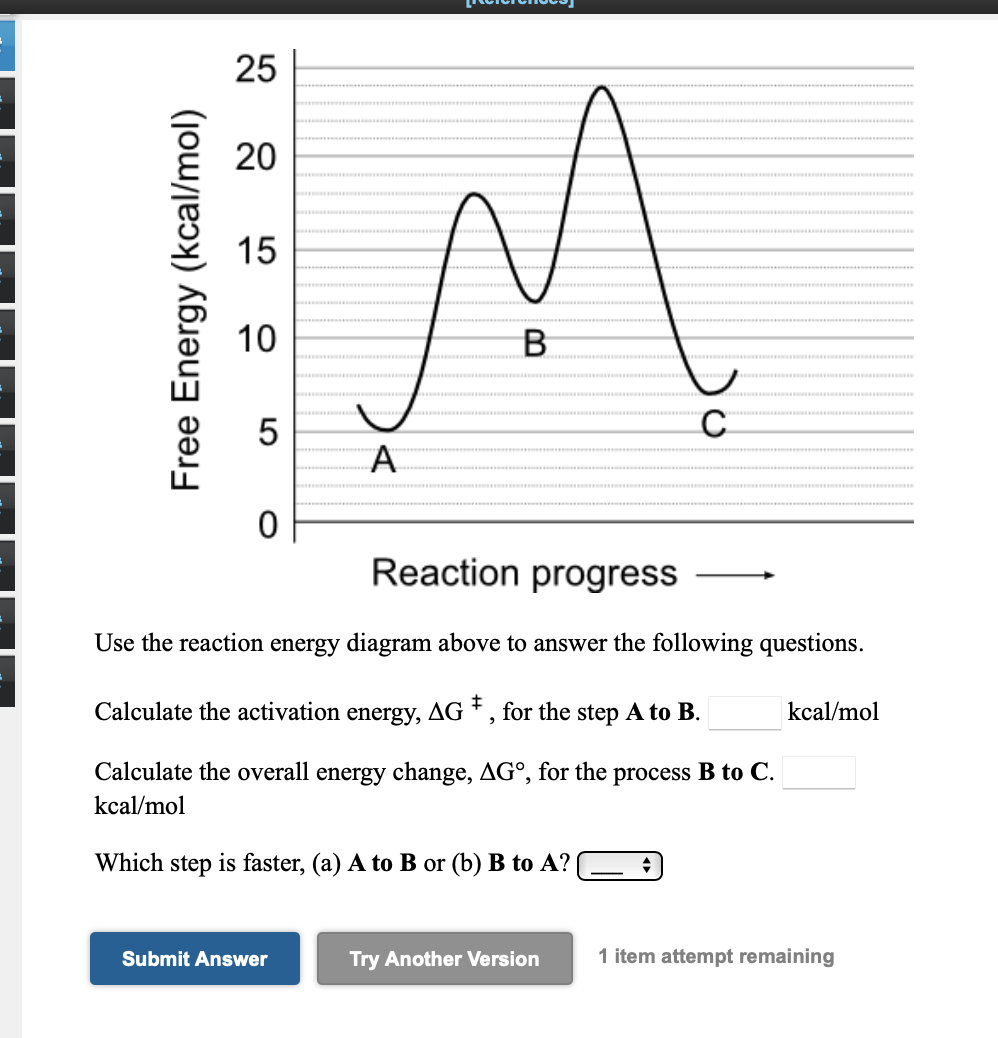
Answered: 25 20 15 10 B 5 C A Reaction progress… | bartleby
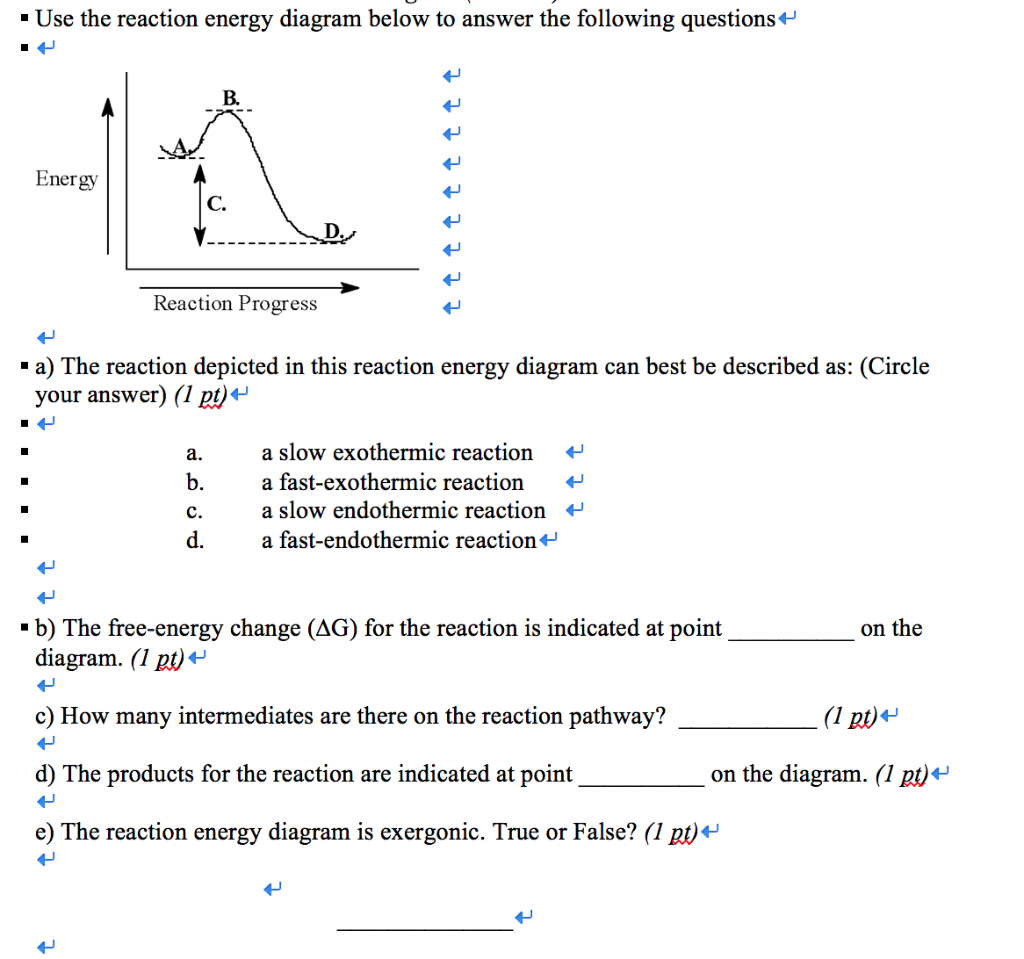
Solved • Use the reaction energy diagram below to answer the ...
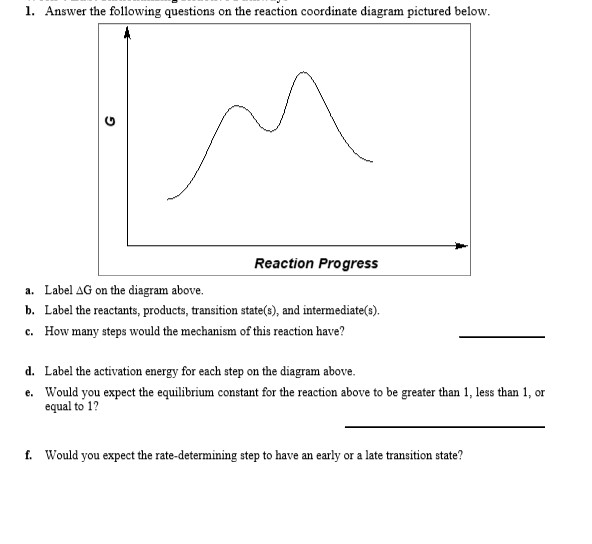
Answered: 1. Answer the following questions on… | bartleby
![Solved] 25 Free Energy (kcal/mol) 20 15 10 B A C D O Reaction ...](https://www.coursehero.com/qa/attachment/11190355/)
Solved] 25 Free Energy (kcal/mol) 20 15 10 B A C D O Reaction ...
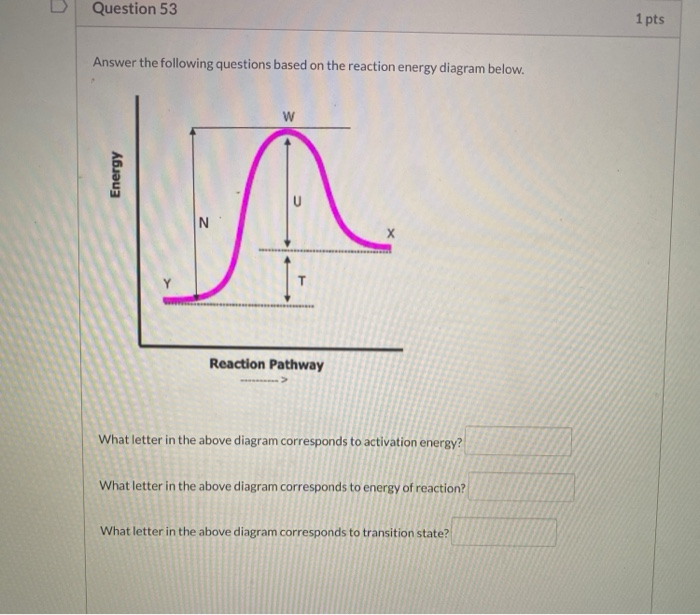
Solved Answer thowing question based on the reaction energy ...
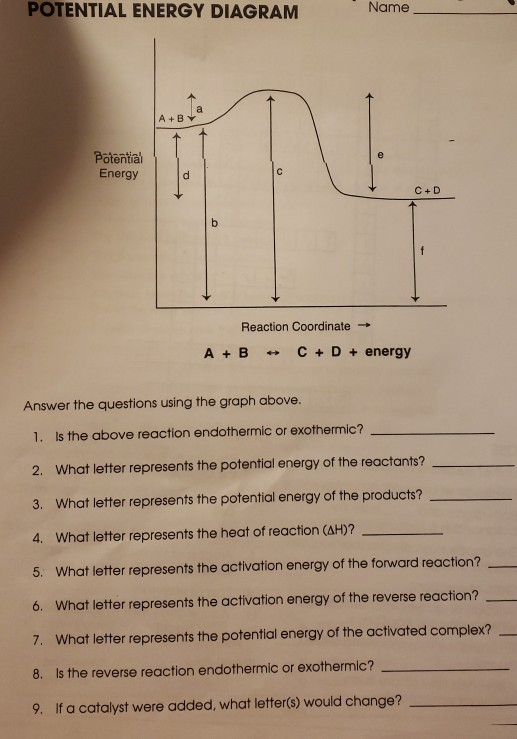
Solved POTENTIAL ENERGY DIAGRAM Name A + B Potential Energy ...
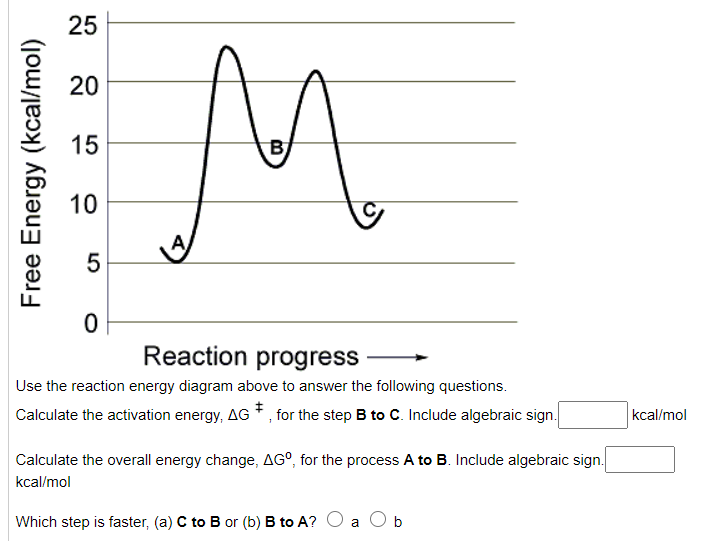
Solved Use the reaction energy diagram above to answer the ...
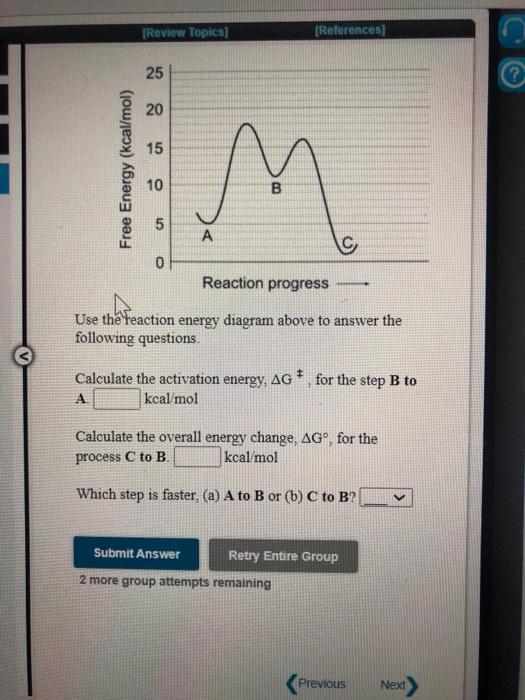
Solved Use the reaction energy diagram above to answer the ...

On a reaction energy profile diagram, how ... | Clutch Prep

ER10 - Temperature, Rate and Potential Energy Diagrams ...

12.1 Energy changes in chemical reactions | Energy and ...
![Solved] 25 20 15 Free Energy (kcal/mol) 10 B A C O Reaction ...](https://www.coursehero.com/qa/attachment/18765983/)
Solved] 25 20 15 Free Energy (kcal/mol) 10 B A C O Reaction ...

Suppose that the reaction A $\longrightarrow$ products is ex ...

Quiz 4 – Potential Energy Diagrams

Potential Energy Diagrams - Chemistry - Catalyst, Endothermic ...

reaction energy diagram question | Ap chem, Potential energy ...
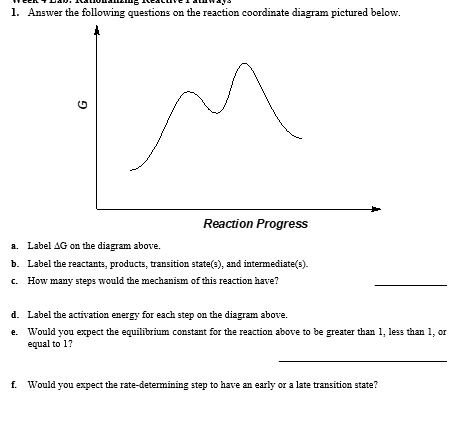
Answered: 1. Answer the following questions on… | bartleby

How can I represent an endothermic reaction in a potential ...

Potential Energy Diagrams

OneClass: Use the reaction energy diagram above to answer the ...
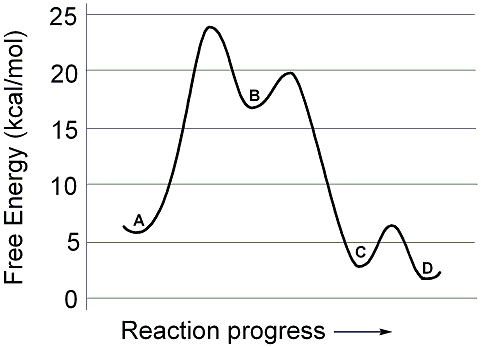
Solved Use the reaction energy diagram above to answer the ...

12.3 Activation energy and the activated complex | Energy and ...

Label the energy diagram for a two-step re... | Clutch Prep
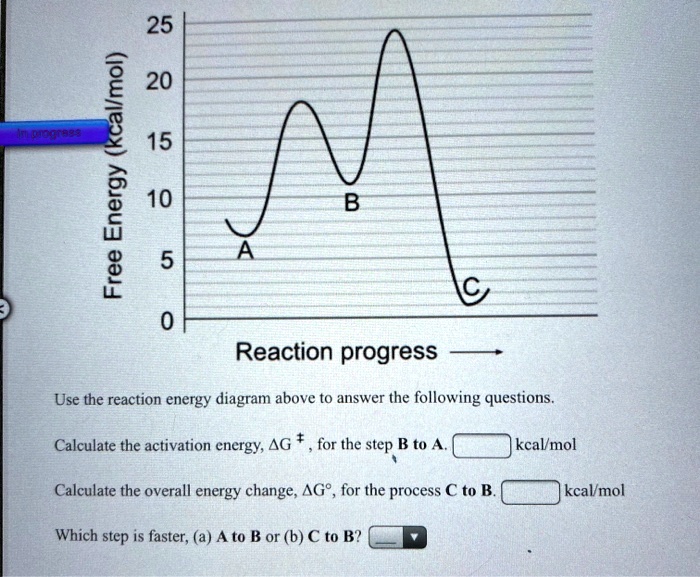
25 kcallmol) 20 15 Energy 10 3 5Reaction progressUse t ...
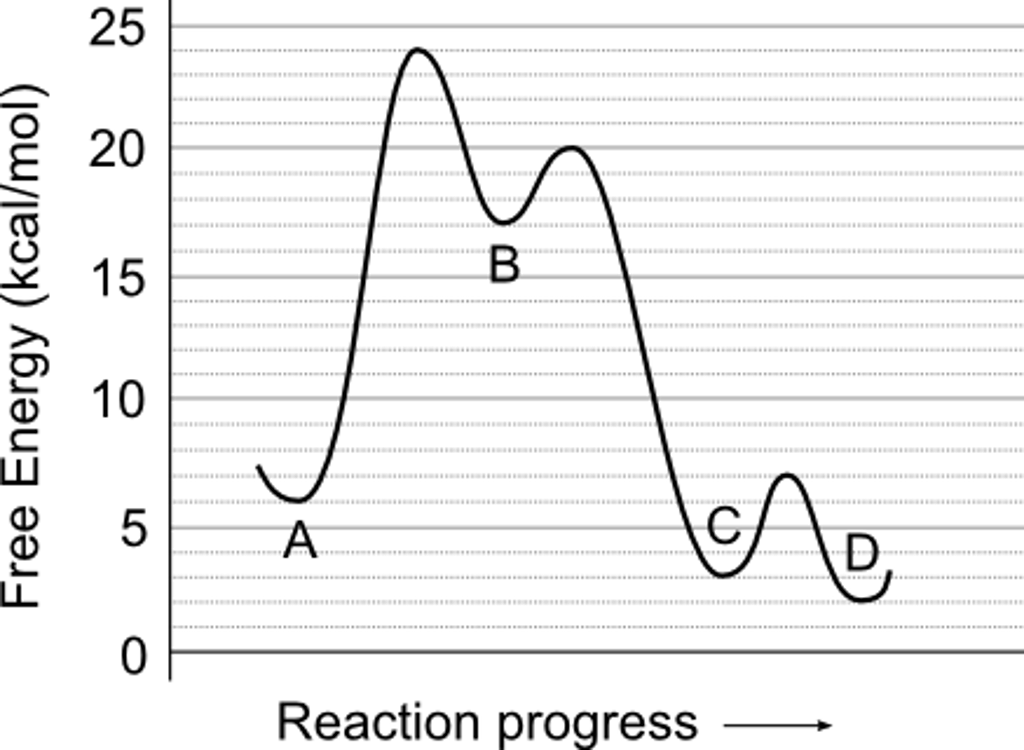
Solved 1) Use the reaction energy diagram above to | Chegg.com

18.4: Potential Energy Diagrams - Chemistry LibreTexts
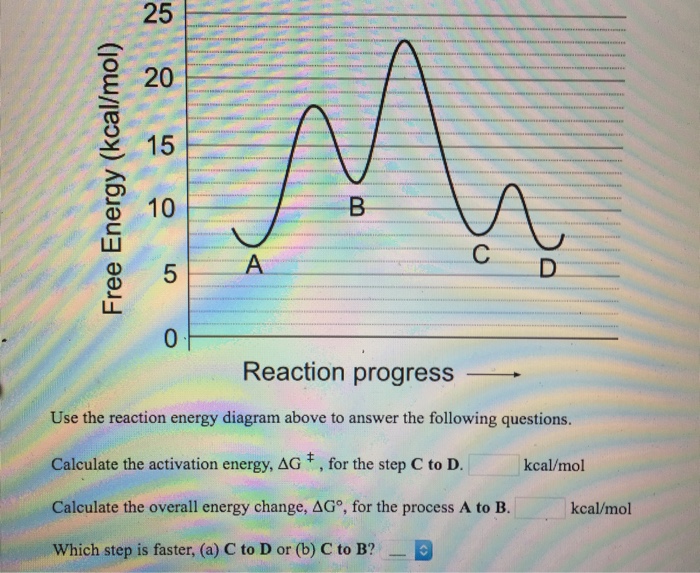
Solved Use the reaction energy diagram above to answer the ...

HELP Activity.dollocator assignment-take&take ...

How to draw the potential energy diagram for this reaction ...

Physics of Uranium and Nuclear Energy - World Nuclear Association

Free Energy (kcal/mol) Reaction progress- Use the rea ...

SN1 Reaction Energy Diagram
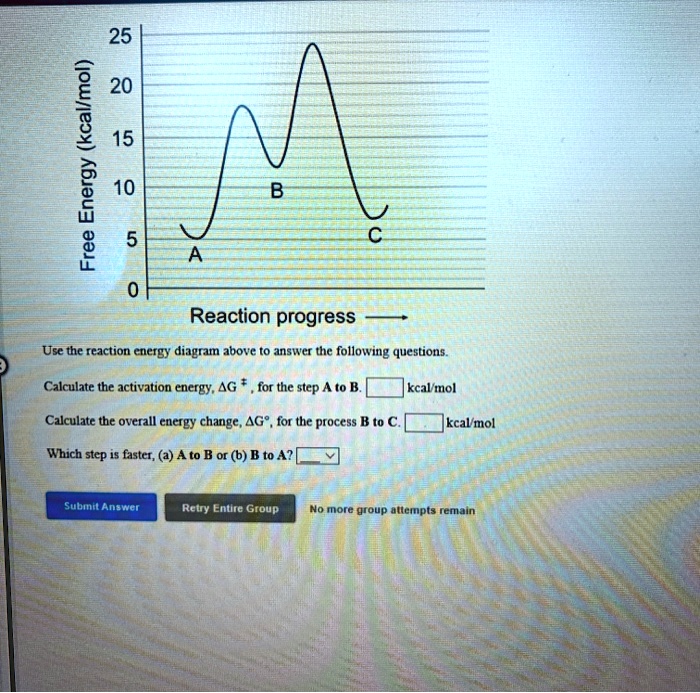
SOLVED:25 20 (kcallmol) 15 Energy 10 Free Reaction progress ...

Activation energy (article) | Khan Academy
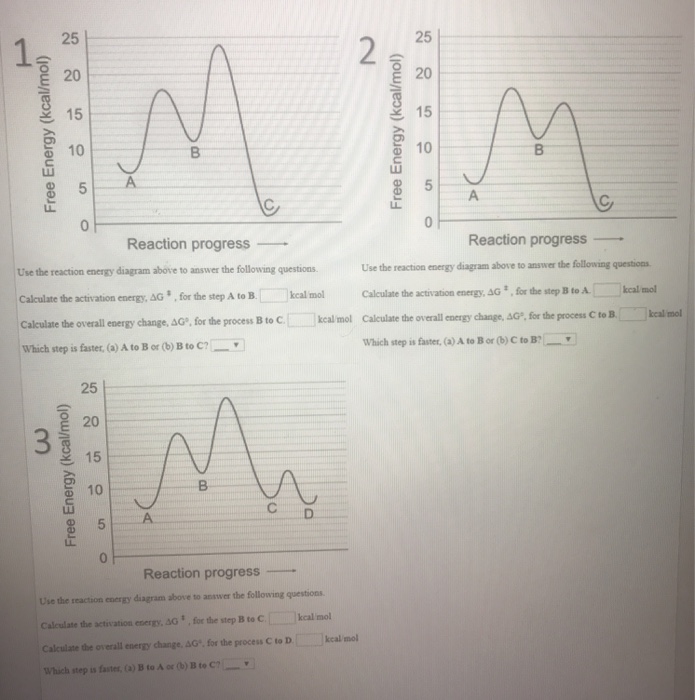
Solved For questions 1, 2, and 3 Use the reaction energy ...
![Solved 4. [2pts] Consider the following energy diagram to ...](https://media.cheggcdn.com/media%2Fe36%2Fe36b3d57-dc8b-4735-aa1a-ec8f972df35b%2Fimage.png)
Solved 4. [2pts] Consider the following energy diagram to ...

Pe Diagram Practice - Kathleen Scrivani | Library | Formative
































![Solved 4. [2pts] Consider the following energy diagram to ...](https://media.cheggcdn.com/media%2Fe36%2Fe36b3d57-dc8b-4735-aa1a-ec8f972df35b%2Fimage.png)

0 Response to "40 use the reaction energy diagram above to answer the following questions."
Post a Comment