37 complete the sentences analyzing the high-pressure phase diagram of ice.
7.1 Introduction: Recall from Chapter 1 that solutions are defined as homogeneous mixtures that are mixed so thoroughly that neither component can be observed independently of the other. Solutions are all around us. Air, for example, is a solution. If you live near a lake, a river, or an ocean, that body of water is not pure H 2 O but most probably a solution. A container of water has been outdoors in cold weather until a 5.0 cm-thick slab of ice has formed on its surface. The air above the ice is at -10 ¡ C. Calculate the rate of formation of ice (in centimeters per hour) on the bottom surface of the ice slab. Take the thermal conductivity of ice to be 0.0040 cal/s . cm .
We’re being asked to determine what three forms of ice present at the triple point marked O. Recall that a phase diagram shows the transition of matter between solid, liquid, and gas phases as temperature and pressure changes. • Critical Point: the point at which the liquid and gas phases coexist. The resulting phase is a supercritical fluid.
Complete the sentences analyzing the high-pressure phase diagram of ice.
during the phase change. Intermolecular Forces. Intermolecular Forces • Calculate the enthalpy change upon converting 1.00 mol of ice at -25 °°°°C to water vapor (steam) at 125 °°°°C under a constant pressure of 1 atm. The specific heats of ice, water, and steam are 2.09 J/g-K, 4.18 J/g-K and 1.84 J/g-K, respectively. PROTOSTAR PHASE:-luminosity much greater than the Sun-radius much larger than the Sun-pressure and gravity are not precisely balanced-energy generated by gravitational contraction MAIN SEQUENCE PHASE:-energy generated by nuclear fusion-lasts about 10 billion years-surface radiates energy at same rate that core generates energy (a) 1, 2, and 3 (b) 4, 5, and 6 (c) 1 and 2 only (d) 4 and 6 only (e) some other combination 11. According to the phase diagram given for Compound Y, what description is correct? (a) At the temperature and pressure at point 4, Y(g) will spontaneously convert to Y(l).(b) At 0 o C and 1200 torr, Y exists as a solid. (c) At the pressure and temperature of point 1, Y(s) will spontaneously convert ...
Complete the sentences analyzing the high-pressure phase diagram of ice.. SENTENCE PATTERN We painted the wall green yesterday . physics. Scientists want to place a 3300 kg satellite in orbit around Mars. They plan to have the satellite orbit a distance equal to 2.2 times the radius of Mars above the surface of the planet. Here is some information that will help solve this problem: mmars = maths A semi-permeable membrane is a type of membrane, which selectively allows the movement of particles or ions via osmosis.. If the molecules are small enough, then they will diffuse across the membrane because the pores of the membrane are small, through which molecules can easily pass.. The semi-permeable membrane can be a biological, synthetic, or polymeric layer that allows the selective ... Complete the sentences analyzing the high-pressure phase diagram of ice. Match the words in the left column to the appropriate blanks in the sentences on the right. The high-pressure phase diagram of ice is shown. Notice that, under high pressure, ice can exist in several different solid forms (Figure 1). Brainly is the knowledge-sharing community where 350 million students and experts put their heads together to crack their toughest homework questions.
Example 2.4-2: Change in temperature with respect to pressure 2-24 Example 2.4-3: Estimation of thermodynamic property 2-26 Example 2.4-4: Heat required to heat a gas 2-27 Chapter 3: Phase Equilibria 3.1 Phase and Pure Substance 3-1 3.2 Phase Behavior 3-4 Example 3.2-1: Specific volume from data 3-7 Phase diagram is a graphical representation of the physical states of a substance under different conditions of temperature and pressure. A typical phase diagram has pressure on the y-axis and temperature on the x-axis. As we cross the lines or curves on the phase diagram, a phase change occurs. In addition, two states of the substance coexist ... Ice V Ice VI Ice VII The three forms of ice present at the triple point Question : u may want to reference (Pages 491-494) Section 11.8 while completing this problem Part A Complete the sentences analyzing the high-pressure phase diagram of ice. In summary, we provide a comprehensive SI ice phase diagram from 200 GPa to 2.5 TPa and up to 3,500 K in the classical limit. At 1,900 K, we find a transition between the CP-SI phase and the P2 1 ...
A phase diagram is a pressure-temperature graph that shows the rangers of temperature and pressure over which each phase is stable. The phase diagram... Q. The triple point of water is 0.0098 °C at 0.00603 atm (4.58 torr). At the triple point, ice, water, and water vapor exist in equilibrium with each... Phase change is often shown in a diagram like the one below: Diagram of water phase changes When a substance is in a solid state, it can absorb a lot of energy in the form of heat until it hits ... Air Pressure Can Crusher - Science Experiment. There are a lot of different ways to crush an ordinary soda can. You can be a super can crusher and crush that empty aluminum can with your foot, in your hands or on your head. However, nothing compares to the fun you'll have performing this soda can implosion experiment from Steve Spangler ... We can use the phase diagram to identify the physical state of a sample of water under specified conditions of pressure and temperature. For example, a pressure of 50 kPa and a temperature of −10 °C correspond to the region of the diagram labeled "ice." Under these conditions, water exists only as a solid (ice).
Label the samples accordingly and complete the two sentences with labels that correctly describe seasonal sea ice melt and seawater freezing and the relationship to salinity. ... water on the salty side of a semipermeable membrane is pushed under high pressure through the membrane to the freshwater side. ... On the lower pie diagram shown below ...
Read the paragraph below and complete the sentences. Stori… Get the answers you need, now! shannonb shannonb 11/19/2015 English High School answered • expert verified Select the correct answer from each drop-down menu. Read the paragraph below and complete the sentences.
diagram the different phases of the cell cycle, labeling the parts of the cell that are pertinent. Labels may include the percentage of the time cells spend in each phase. summarize the following regarding meiosis: Meiosis occurs in sexual reproduction when a diploid cell produces four haploid daughter cells that can mature to become gametes.
Brainly is the knowledge-sharing community where 350 million students and experts put their heads together to crack their toughest homework questions.
This chapter builds on the introduction to the arrangement of particles in materials that was covered in the chapter 'Solids, Liquids and Gases' of the Gr. 6 Matter and Materials curriculum. In Gr. 6, no distinction was made between atoms and molecules. These were grouped together and the generic term 'particle' was used to refer to these ...
Sea ice begins as thin sheets of smooth nilas in calm water (top) or disks of pancake ice in choppy water (2 nd image). Individual pieces pile up to form rafts and eventually solidify (3 rd image). Over time, large sheets of ice collide, forming thick pressure ridges along the margins (4 th image). (Nilas, pancake, and ice raft photographs courtesy Don Perovich, Cold Regions Research and ...
Phase Diagrams. The figure below shows an example of a phase diagram, which summarizes the effect of temperature and pressure on a substance in a closed container. Every point in this diagram represents a possible combination of temperature and pressure for the system. The diagram is divided into three areas, which represent the solid, liquid ...
Rearranging and solving gives: V 2 = 0.300 L×303 K 283 K = 0.321 L V 2 = 0.300 L × 303 K 283 K = 0.321 L. This answer supports our expectation from Charles's law, namely, that raising the gas temperature (from 283 K to 303 K) at a constant pressure will yield an increase in its volume (from 0.300 L to 0.321 L).
The critical point and the orange line in the ice-one phase space refer to the low-density (LDA) and high-density (HDA) forms of amorphous water (ice) . Although generally accepted, the existence of this second, if metastable, critical point is impossible to prove at the present time and is disputed by some [ 200 , 618 , 628 ].
7-26 During the isothermal heat addition process of a Carnot cycle, 900 kJ of heat is added to the working fluid from a source at 400°C. Determine (a) the entropy change of the working fluid, (b) the entropy change of the source, and (c) the total entropy change for the process.7-29 Refrigerant-134a enters the coils of the evaporator of a refrigeration
Millions trust Grammarly's free writing app to make their online writing clear and effective. Getting started is simple — download Grammarly's extension today.
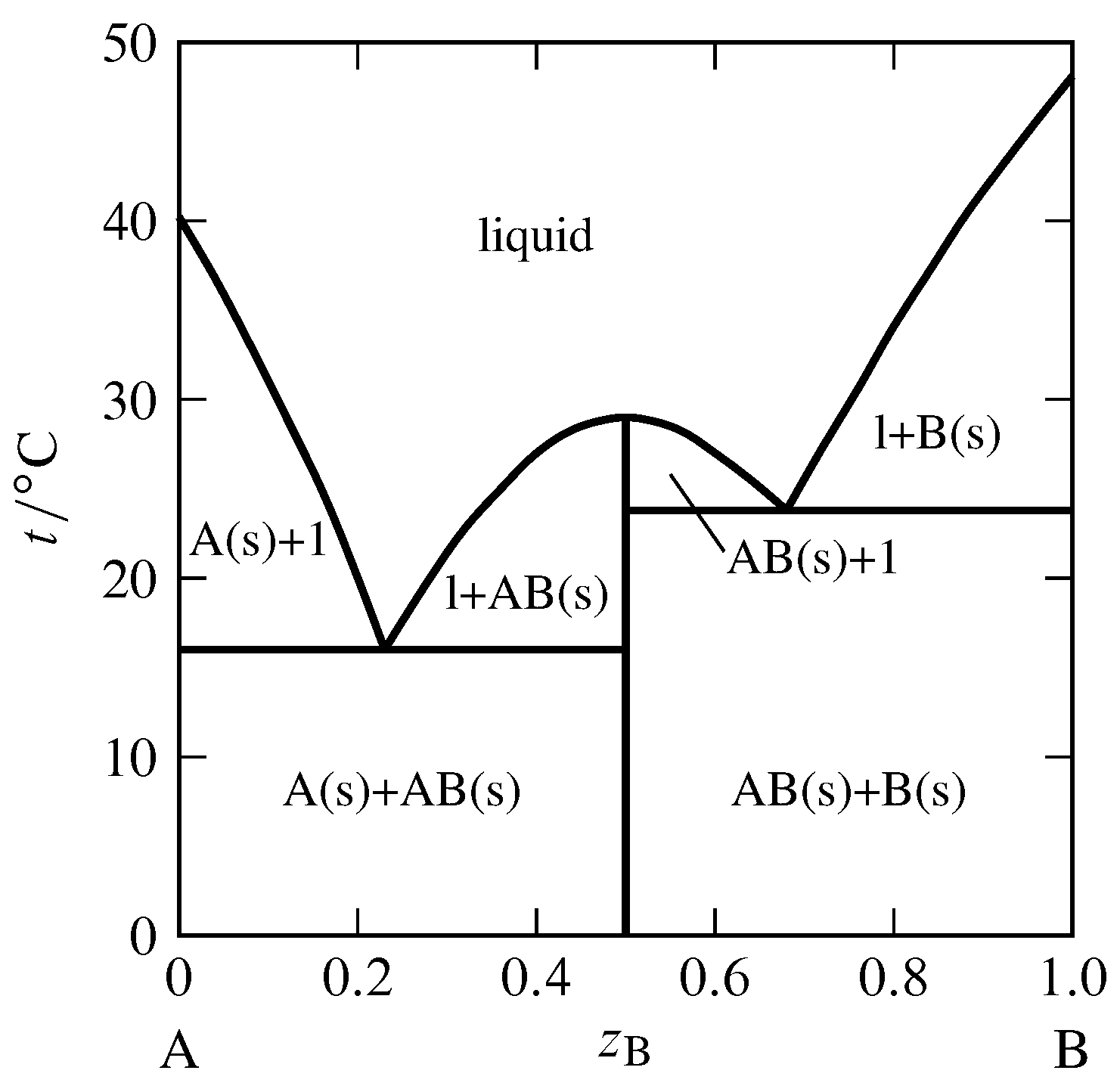
When a diagram is complete, you should be able to place your pencil point at any place on the diagram, and predict what phase or phases are stable under those T-X conditions. Label each field with a label that describes the phase or phases that would be present if your experiment were conducted at the T and X conditions appropriate to that field.
The high pressure phase diagram of Isis shown here I've done the best I could to try to represent this out of a textbook note that under high pressure ice can exist in several different ice solid forms when three wish Three forms of ice are present at the triple point marked Oh, and it's The book points to where I've indicated rate.
To model the next phase, place your back to the sun, and hold the moon straight out in front of you (Earth) but still slightly overhead (see Diagram 2). Notice that it is this inclined orbit position that allows you to see the full half of the moon facing you lit up even when the Earth is between the sun and the moon — Earth's shadow does ...
(a) 1, 2, and 3 (b) 4, 5, and 6 (c) 1 and 2 only (d) 4 and 6 only (e) some other combination 11. According to the phase diagram given for Compound Y, what description is correct? (a) At the temperature and pressure at point 4, Y(g) will spontaneously convert to Y(l).(b) At 0 o C and 1200 torr, Y exists as a solid. (c) At the pressure and temperature of point 1, Y(s) will spontaneously convert ...
PROTOSTAR PHASE:-luminosity much greater than the Sun-radius much larger than the Sun-pressure and gravity are not precisely balanced-energy generated by gravitational contraction MAIN SEQUENCE PHASE:-energy generated by nuclear fusion-lasts about 10 billion years-surface radiates energy at same rate that core generates energy
during the phase change. Intermolecular Forces. Intermolecular Forces • Calculate the enthalpy change upon converting 1.00 mol of ice at -25 °°°°C to water vapor (steam) at 125 °°°°C under a constant pressure of 1 atm. The specific heats of ice, water, and steam are 2.09 J/g-K, 4.18 J/g-K and 1.84 J/g-K, respectively.

0 Response to "37 complete the sentences analyzing the high-pressure phase diagram of ice."
Post a Comment