37 diagram of exothermic reaction
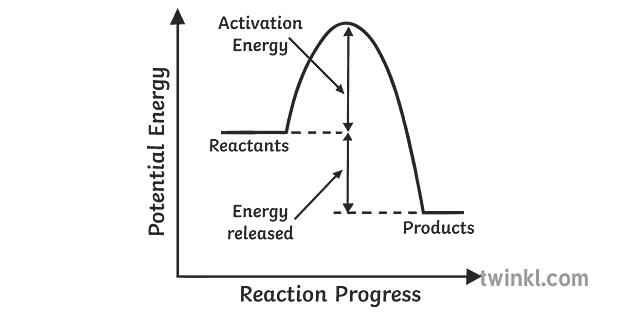
A chemical reaction that involves the release of energy in the form of heat or light is known as an exothermic reaction. When carbon burns in oxygen to make carbon dioxide, for example, a large amount of heat is produced. C + O2 → CO2 (Carbon) (Oxygen) (Carbon dioxide) In this type of reaction, energy (in the form of heat, sound or light) is released when the reactants break apart. Heat energy can be picked up by the area surrounding the products. This means that there was more energy in reactants than in the products. Endothermic Reaction.
Endothermic and exothermic reactionsPaul Andersen explains how heat can be absorbed in endothermic or released in exothermic reactions. An energy diagram can be used to show energy movements in these reactions and temperature can be used to measure them macroscopically.

Diagram of exothermic reaction
Exothermic reactions are opposed to endothermic reactions which involve the absorption of energy from the surrounding in the form of heat. Exothermic chemical reactions transfer the energy in the surrounding area. In the presence of water, a strong acid will dissociate quickly and release heat, therefore it is an exothermic reaction. Exothermic Reaction Energy Profile Diagram. Here are a number of highest rated Exothermic Reaction Energy Profile Diagram pictures on internet. We identified it from obedient source. Its submitted by government in the best field. In exothermic reactions H 2 is lower than H 1. Examples: Manufacture of ammonia in the Haber process; i.e. N 2(g) + 3H 2(g) → 2NH 3(g); ΔH=-46Kjmol-1; Dissolving sodium hydroxide pellets in water, The temperature of the resulting NaOH (aq) is higher than the temperature of water at room temperature.; This implies the internal temperature of products is lower than the reactants' original ...
Diagram of exothermic reaction. The arrow in the enthalpy diagram points from reactants to products. The direction of the arrow in an enthalpy diagram indicates whether a reaction is exothermic or endothermic. Enthalpy, H, increases up the y-axis. In an endothermic reaction, the reactants will be at the bottom of the enthalpy diagram. reaction. Exothermic is when delta H is negative; energy is removed overall, so the ending energy is lower than the starting energy. In this endothermic diagram, the products have a higher enthalpy than the... Exothermic Reaction Energy Profile Diagram. Here are a number of highest rated Exothermic Reaction Energy Profile Diagram pictures on internet. We identified it from honorable source. Its submitted by processing in the best field. a diagram used to show energy changes in a reaction 3.The products of a particular reaction have more energy than the reactants. Which type of reaction has occurred?(1 point) exothermic endothermic convection radiation 4.Which type of reaction is occurring during a fireworks show? How do you know?(1 point) It is an exothermic reaction.
Exothermic Process - Reactions in which the reactant products are having much higher energy than the reaction products and the change in enthalpy remains always negative (H <0). The following diagram will help us to understand the two processes: Endothermic Reaction. Exothermic Reaction. Bond enthalpy: Hydrogenation of a Propene We receive this kind of Energy Level Diagram For Exothermic Reaction graphic could possibly be the most trending subject later than we allocation it in google improvement or facebook. We attempt to introduced in this posting past this may be one of fantastic hint for any Energy Level Diagram For Exothermic Reaction options. The activation energy for a reaction is illustrated in the potential energy diagram by the height of the hill between the reactants and the products. What is energy level diagram. Click to see full answer. Even exothermic reactions such as burning a candle require energy input. Then the rocket is launched and explodes high in the sky. K Ae-E a RT. The potential energy diagram for a reaction starts at 380 kJ and ends at 100 kJ. What type of reaction does the diagram best represent? Exothermic reaction Endothermic reaction Reaction between two solids Reaction between two liquids. Categories English. Leave a Reply Cancel reply.
Answers: 3 on a question: The diagram shows the potential energy changes for a reaction pathway. (8 points) A curved lined graph is shown. The y axis of the graph has the title Potential Energy. The x axis of the graph has the title Reaction Pathway. The graph curve begins at a higher level and ends at a slightly lower level. A vertical line labeled A, starting from the x axis till the ... From the given energy diagram we conclude that this is an exothermic reaction diagram. Thus, the enthalpy of reaction is, -20 kJ. The enthalpy of reaction is the amount of heat that is required or to be released in order to convert the reactants to the products. this can be calculated through the equation, δh = h (products) - h (reactants) Energy diagrams show the level of energy of the reactants and of the products. The bigger the difference between the energy of the reactants and the energy of the products, the more energy is given out or taken in. It is easy to see from an energy level diagram whether the reaction is exothermic or ... Dear student, P …. View the full answer. Transcribed image text: Consider the reaction diagram. This reaction is O exothermic. Energy (kJ/mol) endothermic. What is the heat of reaction? Reaction progress AH kJ/mol.
What type of reaction does the diagram best represent? Exothermic reaction Endothermic reaction Reaction between two solids Reaction between two liquids 1 See answer Advertisement Advertisement kirakosoveox5gp9 is waiting for your help. Add your answer and earn points.
An Exothermic reaction is a chemical reaction that involves the release of energy in the form of heat or light. These reactions are the opposite of endothermic reactions and can be expressed in a chemical equation as follows: Reactants → Products + Energy What is an Exothermic Reaction?
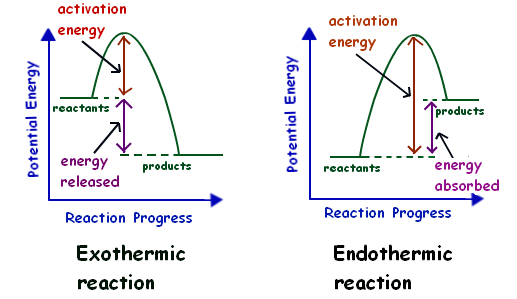
Figure 3: Energy Pro±le Diagrams of Exothermic and Endothermic Reactions Thermochemical Equations The energy change that occurs during a reaction can be included with the equation. The equations that include the energy change are called thermochemical equations. The states of all the species must be included in thermochemical equations.
EQUATIONS &. ENERGY DIAGRAMS. • WE CAN USE AN ENERGY DIAGRAM FOR A VISUAL REPRESENTATION OF THE ENERGY WITHIN. A REACTION.
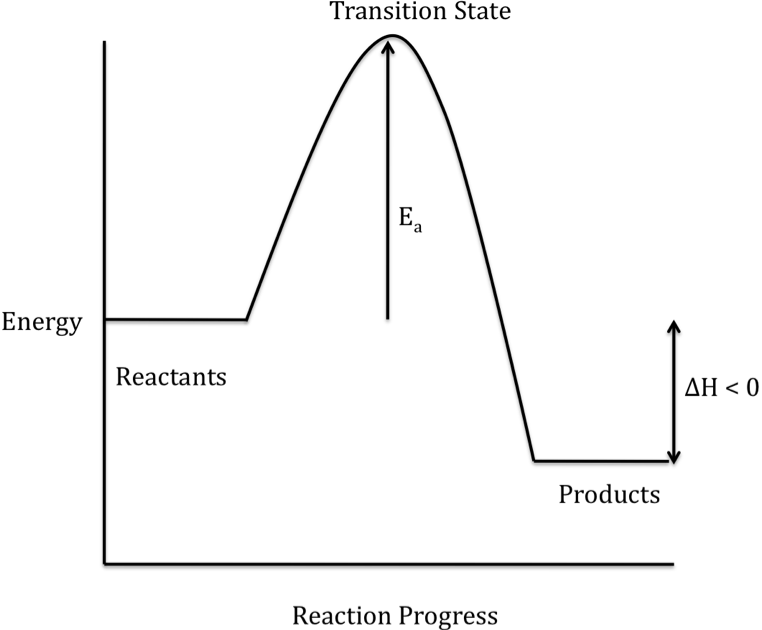
interpret the reaction energy diagram for a single-step process (e.g., use the diagram to decide whether the reaction is exothermic or endothermic). suggest possible transition-state structures for simple one-step processes. assess the likelihood of a reaction occurring at room temperature, given the value of the activation energy ΔG ‡.
The diagram shows the potential energy changes for a reaction pathway. Part 1: Does the diagram illustrate an endothermic or an exothermic reaction? Give reasons in support of your answer. Part 2: Describe how you can determine the total change in enthalpy and activation energy from the diagram and if each is positive or negative.
A chemical reaction or physical change is exothermic if heat is released by the system into the surroundings. Because the surroundings is gaining heat from the system, the temperature of the surroundings increases. The sign of q for an exothermic process is negative because the system is losing heat. Figure 3.14. 2: (A) Endothermic reaction.
Energy level diagram for an Exothermic change. Thermal energy is released by the System into the Surroundings. This results in a lower Enthalpy value of the final state with respect to the initial...
Aanlyze the potential energy diagram of the reaction shown. Which statements about the reaction are true? Select all that apply. The reaction is exothermic The reaction is endothermic The equation shows that 891 kJ of energy are released as a product. The equation shows that 891 kJ of energy are absorbed as a reactant.
Energy Diagrams. Exothermic Reactions. Endothermic Reactions. Example. 6.3 Kinetic Energy, Heat Transfer, and Thermal Equilibrium. 6.4 Heat Capacity and Coffee-Cup Calorimetry. 6.5 Phase Changes and Energy. 6.6 Introduction to Enthalpy of Reaction. 6.7 Bond Enthalpy and Bond Dissociation Energy.
Potential energy diagrams are used to represent potential energy changes that take place as time progresses during chemical reactions. the potential energy diagrams for the exothermic reaction between substances x and y is shown below. the reaction progresses from stage 1 (reactants), through stage 2 (activated complex to), to stage 3 (products.
May 1, 2013 - Explains potential energy diagrams and activation energy.
August 9, 2021 - An exothermic reaction is opposite to an endothermic reaction. In an exothermic reaction, the reactants transform into products and release heat. In the energy level diagram, the enthalpies of the products are lower than that of the reactants. Hence, the enthalpy change is negative (ΔH<0).
Exothermic vs. Endothermic and K. An exothermic reaction occurs when the temperature of a system increases due to the evolution of heat. This heat is released into the surroundings, resulting in an overall negative quantity for the heat of reaction ( q r x n < 0 ). An endothermic reaction occurs when the temperature of an isolated system ...
Conclusion. Both exothermic and endothermic reactions can be classified based on energy transfer between the system and surroundings. The main difference is that exothermic reactions release energy into the surroundings whereas an endothermic reaction absorbs energy from the surroundings. Spread the Differences.
July 14, 2020 - Given the following potential energy diagram for this reaction, determine ΔH and Ea for both the forward and reverse directions. Is the forward reaction endothermic or exothermic?
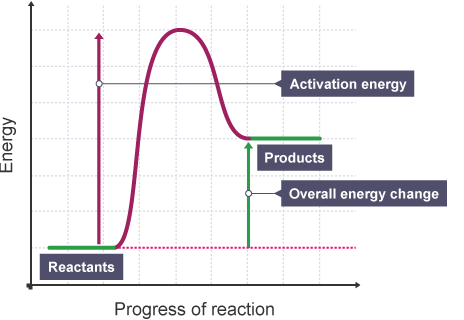
The overall change in energy in a reaction is the difference between the energy of the reactants and products. ... The diagram shows a reaction profile for an exothermic reaction.
The energy changes that occur during ... called a reaction progress curve. A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) ...
Exothermic and Endothermic reaction and their energy profile diagram. Exothermic reaction: The reaction in which heat is liberated /released from the system is called exothermic reaction. For example:- Combustion of methane in oxygen is an exothermic process. Endothermic reaction:
24/10/2021 · The reaction diagrams of exothermic reactions and endothermic reactions are as follows. Endothermic and exothermic reactions can be visually represented by energy-level diagrams like the ones in Figure PageIndex2. As we can see and examine from the graph in an exothermic reaction the reactants are usually at a higher. In this type of reaction energy in the …
Find my revision workbooks here: https://www.freesciencelessons.co.uk/workbooksIn this video, we look at exothermic and endothermic reactions and how these a
Endothermic Reaction Diagram | energy diagram for a catalyzed endothermic reaction ... Chemistry 30 Chemical Kinetics - Potential Energy Diagrams Revisited.
ΔH=ΔU+PΔV The flow of heat (q) at constant pressure in a process equals the change in enthalpy based on the following equation, ΔH=q A relationship between q and ΔH can be defined knowing whether q is endothermic or exothermic.
This is a step-by-step discussion on how to interpret a potential energy diagram of an exothermic reaction. #narcademyCorrection: Ea of the forward reaction ...
Energy level diagrams for exothermic reactions In an exothermic reaction, reactants have more energy than the products . The difference between these two energy levels is the energy released to the surroundings, shown as a vertical drop from a higher to a lower level. Because the reactants have more energy than the products they are less stable.
Aug 28, 2021 — From an energy level diagram, we can determine the following: 1. Is the reaction endothermic or exothermic? 2. Does the product have more energy ...
In this video, I go over how to properly label and explain a reaction mechanism diagram which is also referred to as an energy diagram or energy graph. I'll ...
STUDY CARD AVAILABLE- SEE CONTACT
Professor Patricia Shapley, University of Illinois, 2012
(a) Calculate the activation energy for the reverse reaction. (b) Is the reaction exothermic or endothermic? Explain. (c) Sketch the energy profile diagram for the reaction with and without catalyst. Answer PRACTICE QUESTION 16 Consider the following reaction that occurs between hydrochloric acid, HCl, and zinc
July 9, 2019 -
Here are a number of highest rated Exothermic Reaction Energy Profile pictures upon internet. We identified it from honorable source. Its submitted by management in the best field. We acknowledge this nice of Exothermic Reaction Energy Profile graphic could possibly be the most trending topic subsequently we allowance it in google pro or facebook.
Draw energy profile diagrams for exothermic and endothermic reactions. An exothermic reaction A to B has an activation energy of X kJ mol^(-1) of A. If the energy change in the reaction is Y kJ, then the activation energy of the backward reaction will be 131209637 000+ 1.4 k+
Exothermic reactions in solution give out energy and the temperature increases, while endothermic reactions take in energy and the temperature decreases. Bonds are broken and made in reactions. ... An energy level diagram shows the energy of the reactants and products, and the difference in ...
A.) It is an endothermic reaction. Heat must be added to light the fuses of the fireworks. B.) It is an endothermic reaction. Fireworks are bright, indicating a release of light energy. C.) It is an exothermic reaction. Fireworks are bright, indicating a release of light energy. *** D.) It is an exothermic reaction.
Exothermic reactions usually release heat and entail the replacement of weak bonds with stronger ones. The term is often confused with exergonic reaction, which IUPAC defines as "... a reaction for which the overall standard Gibbs energy change ΔG⚬ is negative."
Which potential energy diagram represents an exothermic reaction? Potential Energy Potential Energy non Reaction coordinate A) Reaction coordinate B) Reaction coordinate C) Reaction coordinate D) Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. a a b b с c d d Which potential energy diagram ...























0 Response to "37 diagram of exothermic reaction"
Post a Comment