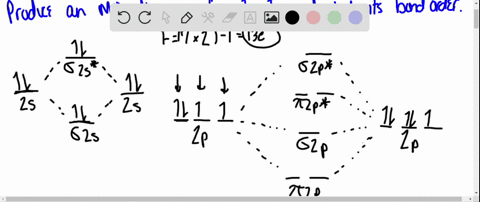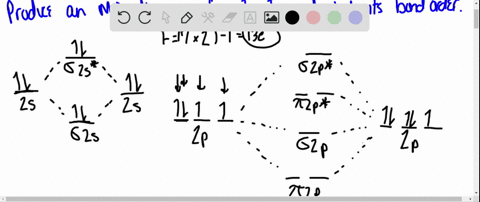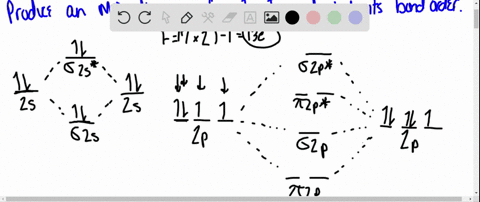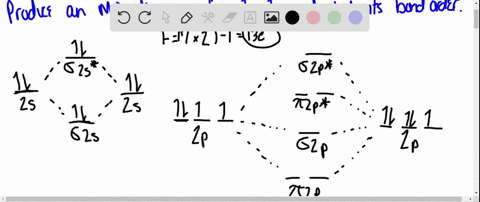
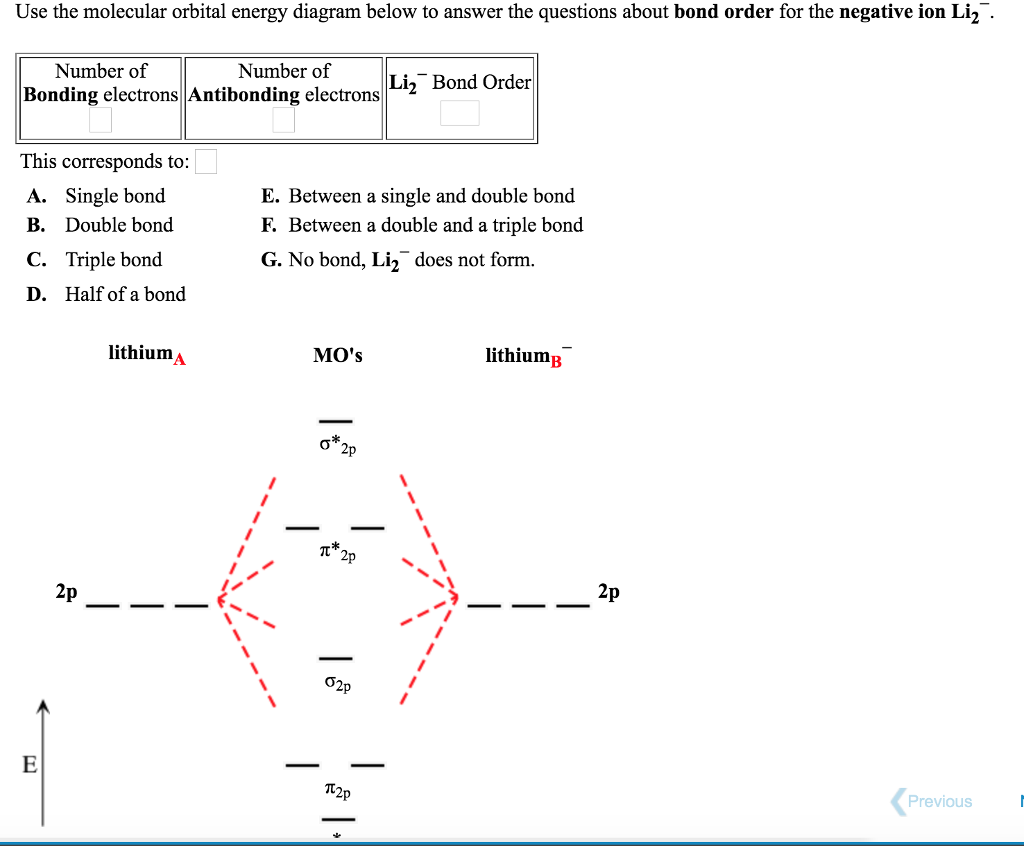
40 use the drawing of the mo energy diagram to predict the bond order of li2+.
Use the drawing of MO energy diagram to predict the bond order ofLi2+ and Li2- . Do youexpect Li2+ to exist in the gas phase? Question: Use the drawing of MO energy diagram to predict the bond order ofLi2+ and Li2- . Do youexpect Li2+ to exist in the gas phase? https://imgur.com/a/LDNi9o4 Just a bad lil drawing but remember when the strat was to put the furthest hobgoblin into status so he wouldn’t one shot the shield carrier
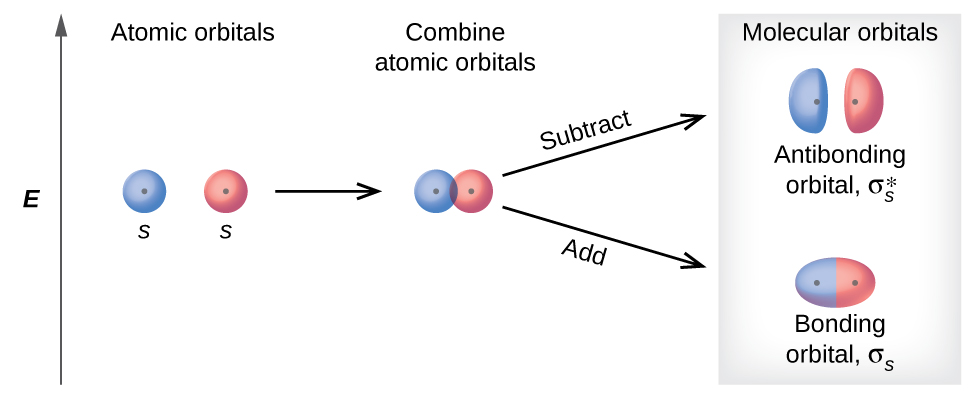
This MO diagram depicts the molecule H2, through the contributing AOs ~ above the external sandwiching the MO. The bonding level (lower level) is totally occupied. A link order of one is acquired by use the formula above, describe a steady bond. \textBond Order = \frac2 (\textbonding electrons)-0(\textanti-bonding\ e-)2 = 1
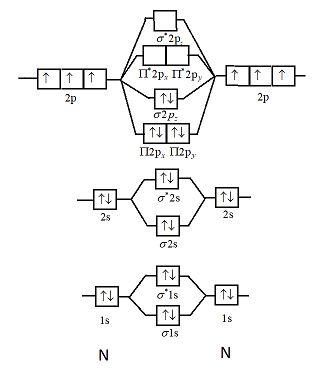
Use the drawing of the mo energy diagram to predict the bond order of li2+.
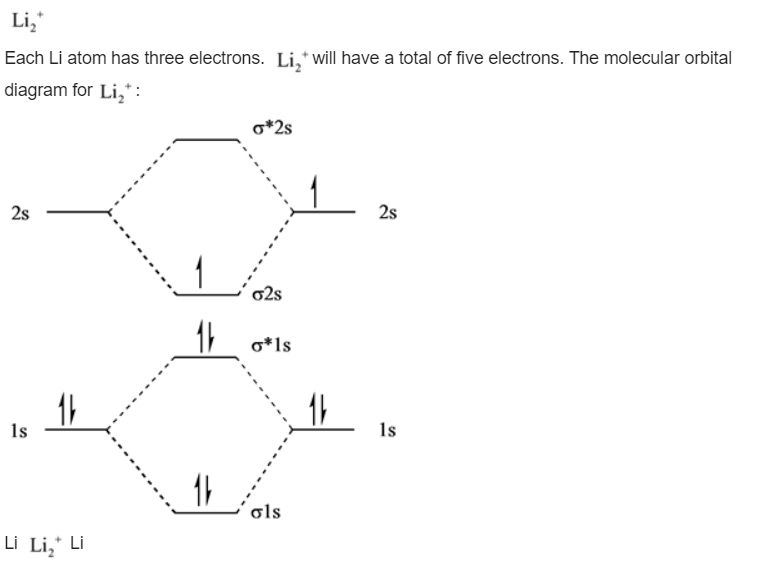
Molecular orbital diagram for nitrogen monoxide, the nitrosyl cation and the nitrosyl anion 1 Order of filling of molecular orbitals in heteronuclear diatomic molecules such as CO. Part A. Use the drawing of the MO energy diagram to predict the bond order of Li2+. Express the bond order as an integer or fraction. Part B. We’re being asked to determine the bond order of Li2–. For this, we need to do the following steps: Step 1: Calculate the total number of valence electrons present. Step 2: Draw the molecular orbital diagram. Step 3: Calculate the bond order of the molecule/ion. Recall that the formula for bond order is: Bond Order = 1 2 [ # of e - in ... Except Microsoft Visio
Use the drawing of the mo energy diagram to predict the bond order of li2+.. Q. Draw the MO energy diagram for CO on your own, then use it to predict the bond order for the molecule. (Use the energy ordering of O2. (Use the energy ordering of O2. Q. Molecular nitrogen, carbon monoxide, and cyanide ion are isoelectronic. Solution for Use the drawing of MO energy diagram for CO to predict the bond order. (Use the energy ordering of O2. ) Chemistry questions and answers. Part A Use the drawing of the MO energy diagram to predict the bond order of Li2+. Express the bond order as an integer or fraction. Part B Use the drawing of the MO energy diagram to predict the bond order of Li2?. Express the bond order as an integer or fraction. Part C Which molecules are predicted to exist ... In a world where the use of magic typically means drawing power from your own body heat, a particularly crafty mage discovers a way to use a controlled nuclear fission reaction as a source of energy. What follows is an epic, action packed fight for survival. In the world of Fables, when a Fable is born a Fable becomes the very embodiment of the magic that created it. The Fable game book is available here on Amazon US, Amazon UK, Barnes and Noble US, and Barnes and Noble UK. Here's the cover: ...
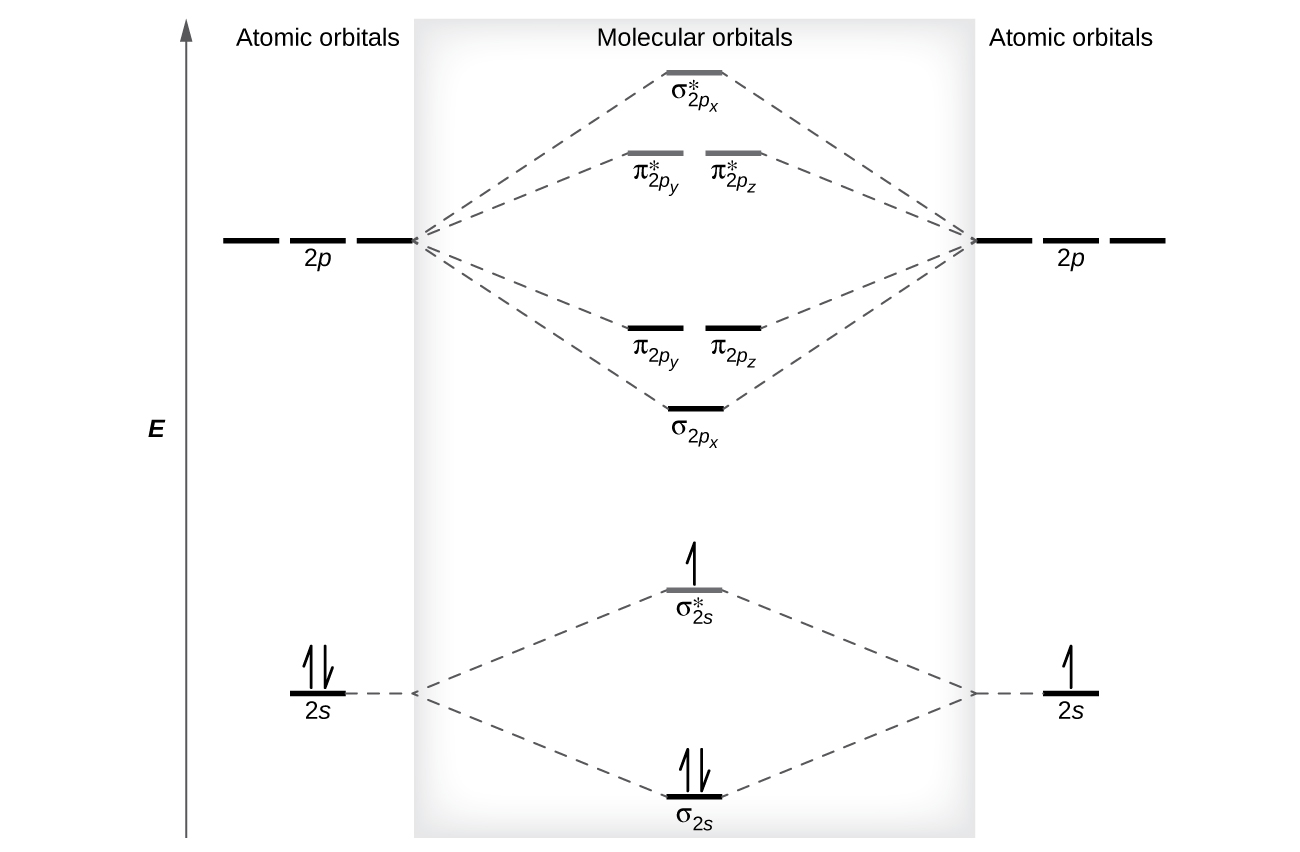
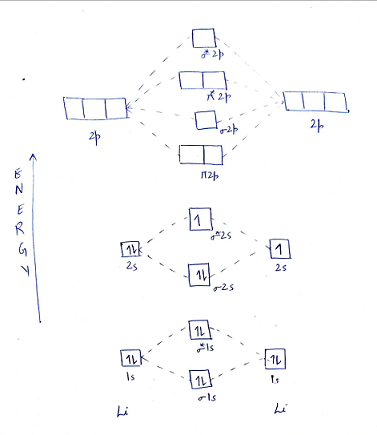
A molecular orbital explicitly describes the spatial distribution of a single electron orbitals, and σ∗. 1s is higher in energy. Draw this out using an energy level diagram: 2 He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. 2,. H−.A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical ... Hi, ​ I'm struggling to understand why, in the following ML4 sigma-only complex, that the metal AO T2 set rises in energy while there is no bonding counterpart to it. ​ I appreciate the rationale in terms of crystal field theory etc but unsure how it's justified in the case of merely looking at symmetry-matching AOs and MOs. ​ [https://imgur.com/a/cNhkgjd](https://imgur.com/a/cNhkgjd) ​ Note how the T2 symmetry d-orbitals rise in energy to be a... Okay, so this problem test our understanding of the molecular arbiters. They asked us to draw the molecular arbitral diagram for the elysium two plus and we see um two miners. So before we draw the diagram first we need to know the electron configurations of the issue. Alicia electron configuration we know is what has to and to s warm. So actually each lithium atom has to up tools that is ... MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
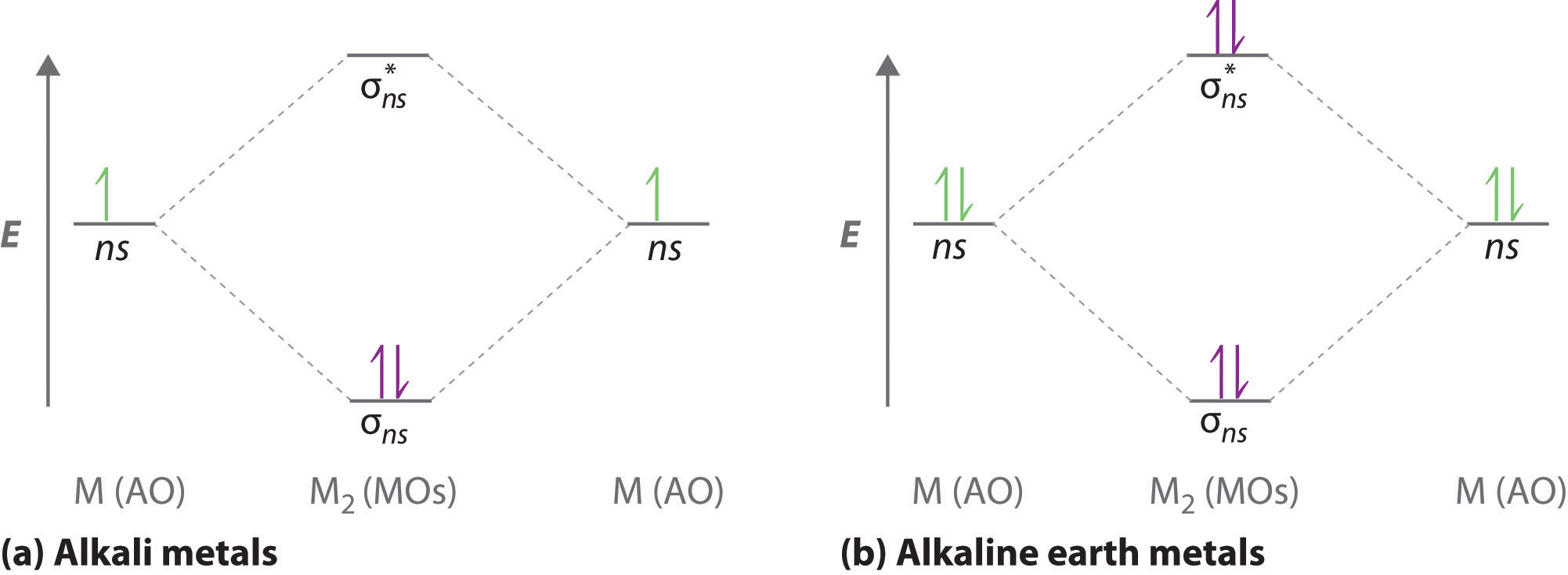
Use simple LCAO (linear combination of atomic orbitals) MO theory. Li(0) 2s^1 Overlap of the two 2s AOs results in a . σ bonding MO that is lower in energy than the constituent 2s AOs and an antibonding σ* MO that is at a higher energy than the 2s AOs. Li2 is more stable than Li+ 2, because the bond is (hypothetically) stronger (probably gas-phase). Here we consider the molecular orbital diagram (MO) of Li2: The bond order can be calculated in a simple manner. Just take electrons that are in each MO, and. for each electron in a bonding MO, it adds 0.5 to the bond order, because more bonding ... Construct a "molecular orbital diagram" of the kind shown in this lesson for a simple diatomic molecule, and indicate whether the molecule or its positive and negative ions should be stable. Part A. Use the drawing of the MO energy diagram to predict the bond order of Li2+. Express the bond order as an integer or fraction. Part B. Use the ... Find step-by-step Chemistry solutions and your answer to the following textbook question: Use MO diagrams and the bond orders you obtain from them to answer: (a) Is Be2+ stable? (b) Is Be2+ diamagnetic? (c) What is the outer (valence) electron configuration of Be2+?.
Q. Draw an MO energy diagram and predict the bond order of Li2+and Li2- . Do you expect these molecules to exist in the gasphase? Do you expect these molecules to exist in the gasphase? See all problems in MO Theory: Bond Order
Answer to Draw an MO energy diagram and predict the bond order of Be2+ and Be2−. Do you expect these molecules to exist in the. Even rather simple molecular orbital (MO) theory can be used to predict which we start reading from the bottom of the diagram because this is how MO diagrams are constructed, Diberyllium, Be2, has a bond order of zero and is unknown.
Science. Chemistry. Chemistry questions and answers. Use the drawing of MO energy diagram to predict the bond order of Li2+ and Li2. Part A Do you expect Li2+ to exist in the gas phase? ANSWER: O yes O no Not completed before the time limit Part B to exist in the gas phase? Do you expect Li2 ANSWER: yes O no Not completed before the time limit.
Problem: Use an MO diagram to find the bond order and predict whether H 2− exists. Problem. : Use an MO diagram to find the bond order and predict whether H 2− exists. FREE Expert Solution. Show answer. Answer: 90% (150 ratings) play-rounded-fill. play-rounded-outline.
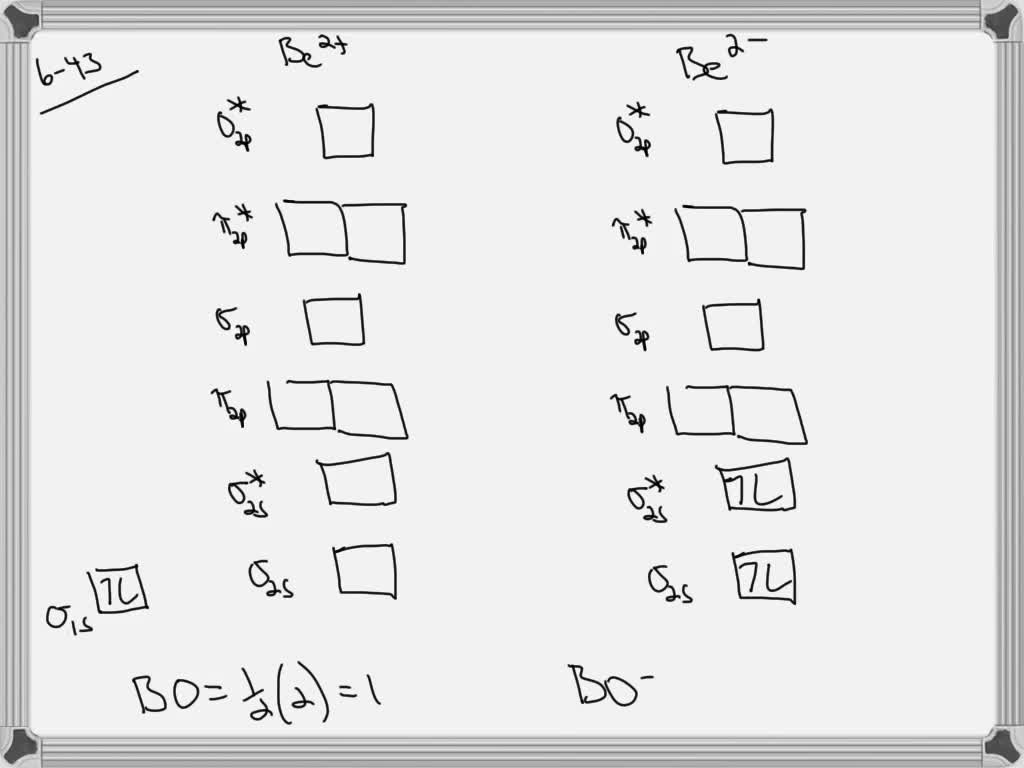
draw an mo energy diagram and predict the bond order of be2 and be2 do you expect these molecules to
This is the molecular orbital diagram for the homonuclear diatomic Be2+, . electrons would be in a bonding orbital, we would predict the Li2 molecule to be . Learn to draw molecular orbital electron configuration energy diagrams. molecular orbital electron configuration diagram for Li2 (Figure "Molecular orbital.
Use the drawing of the MO energy diagram to predict the bond order of Li2+, and use the drawing of the MO energy diagram to predict the bond order of Li2−. Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (30 ratings)
Solution for Draw an MO energy diagram and predict the bond order of Li2 + and Li2 -. Do you expect these molecules to exist in the gas phase?
Welcome to Sarthaks eConnect: A unique platform where students can interact with teachers/experts/students to get solutions to their queries. Students (upto class 10+2) preparing for All Government Exams, CBSE Board Exam, ICSE Board Exam, State Board Exam, JEE (Mains+Advance) and NEET can ask questions from any subject and get quick answers by subject teachers/ experts/mentors/students.
Answer to Draw a molecular orbital energy diagram for Li2. Li2+ is more stable than Li2− because Li2− has more numbers of antibonding electrons. thus the order is Li 2 >Li 2 + >Li 2 - The instantaneous reaction rate is always equal and constant. Molecular electron configuration for o2 σ2σ2σ2π4π2 we can also calculate the oo bond order.
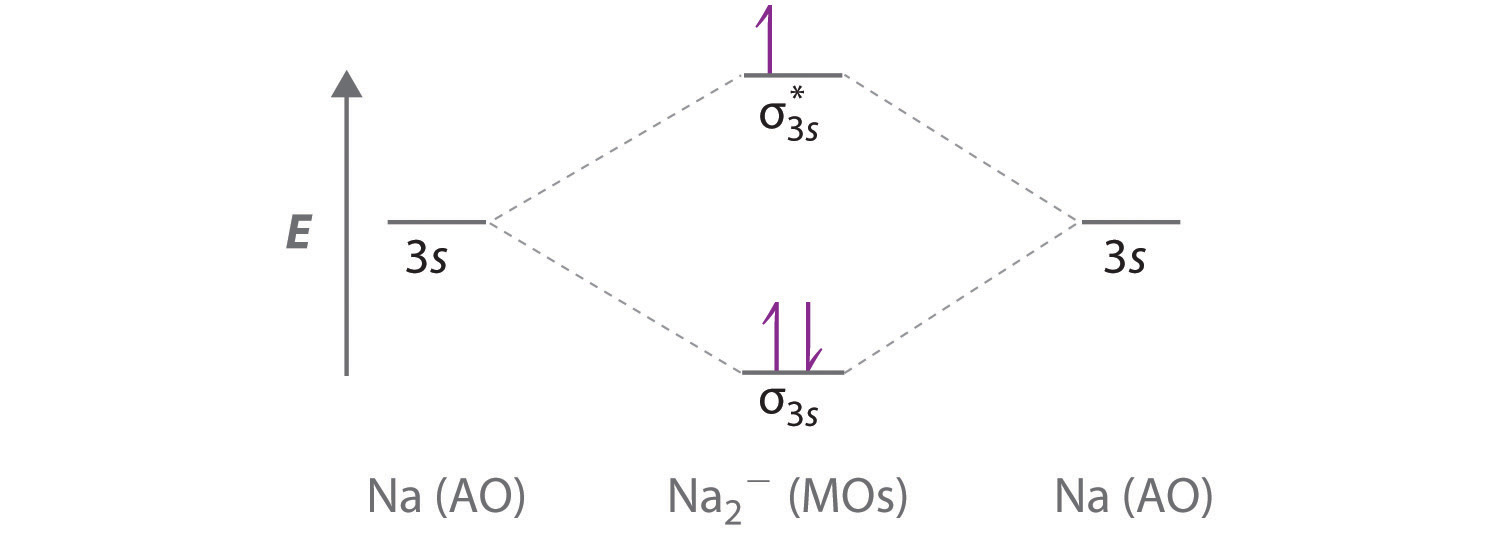
Use a qualitative molecular orbital energy-level diagram to predict the valence electron configuration, bond order, and likely existence of the Na 2 − ion. Given: chemical species. Asked for: molecular orbital energy-level diagram, valence electron configuration, bond order, and stability. Strategy:
We are being asked to draw the MO energy diagram of Li 2 + and Li 2-then predict which will exist in the gas phase.. We will do the following steps. Step 1: Calculate the total number of valence electrons present. Step 2: Draw the molecular orbital diagram. Step 3: Calculate the bond order of the molecule/ion. Recall that the formula for bond order is:. Bond Order = 1 2 [# of e- in bonding MO ...
Except Microsoft Visio
We’re being asked to determine the bond order of Li2–. For this, we need to do the following steps: Step 1: Calculate the total number of valence electrons present. Step 2: Draw the molecular orbital diagram. Step 3: Calculate the bond order of the molecule/ion. Recall that the formula for bond order is: Bond Order = 1 2 [ # of e - in ...
Molecular orbital diagram for nitrogen monoxide, the nitrosyl cation and the nitrosyl anion 1 Order of filling of molecular orbitals in heteronuclear diatomic molecules such as CO. Part A. Use the drawing of the MO energy diagram to predict the bond order of Li2+. Express the bond order as an integer or fraction. Part B.















0 Response to "40 use the drawing of the mo energy diagram to predict the bond order of li2+."
Post a Comment