38 lewis dot diagram for nh3
› 37409050 › general_chemistry_pdf(PDF) general-chemistry.pdf | Sumit Banerjee - Academia.edu Academia.edu is a platform for academics to share research papers. NH3 Lewis Structure - Lewis Dot Structure | Chem Helps NH3 Lewis Dot Structure To write the NH3 Lewis Structure, we need to understand the formation of NH3. The most important feature of this bond, also called electron pair bond, is that the electrons are held tightly and shared equally (jointly) by neighboring atoms. Some element atoms form a more stable structure by sharing one…
Nh3 Lewis Dot Structure - ViralListClub.com Lewis Dot Diagram Of Nh3 The Lewis structure of ammonia NH3 would be three hydrogen atoms bonded to a nitrogen atom in the middle with a lone pair of electrons. There are 8 valence electrons available for the Lewis structure for NH 3.
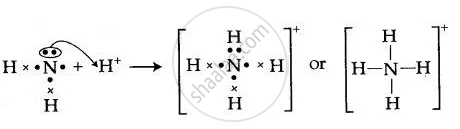
Lewis dot diagram for nh3
How is the Lewis dot diagram for nh3 determined? - Quora What is the Lewis structure of NH4NO3? NH4NO3 is an ionic compound made up of two poly atomic ions, NH4+ and NO3- . NH4+ has 8 valence electrons with the N in the center with sp3 hybridization (tetrahedral) with 4 H atoms bonded to it using the 8 electrons. NO3- has 24 valence electrons with the N in the center and the 3 O atoms bonded to it. Lewis Dot Diagram Nh3 - Novocom.top dot diagram nh3 lewis student diagrams which drew shown students nor neither alqurumresort flordelis says . î Lewisî î Dotî î Diagramî For î Nh3î exatin info . lewis nh3 structure ammonia structures trigonal pyramidal nh sundin dimensional molecular three edu forces uwplatt . What is the electron dot structure for nh3? The electron-dot structure of NH3 places one pair of nonbonding electrons in the valence shell of the nitrogen atom. This means that there are three bonded atoms and one lone pair for a coordination number of four around the nitrogen, the same as occurs in H2O. The Lewis dot structure for ammonia, NH3. Furthermore, what is the shape of nh3 ...
Lewis dot diagram for nh3. quizlet.com › 195082504 › chem-flash-cardschem Flashcards - Quizlet The mass number is the number of dots in the Lewis dot structure for any element. The Lewis dot structure is used to keep track of the valence electrons for each atom. Give the symbol of the element in Group 6A, Period 3. NH3 Lewis Structure, Geometry, and Hybridization ... Draw the lewis diagram as below: Geometrical Structure of the Ammonia (NH3) The bond angle among the hydrogen-nitrogen-hydrogen atoms (H-N-H) is 107°. It is clear to understand that the geometrical structure of NH3 will be bent. topblogtenz.com › nacl-lewis-dot-structure-polarSodium chloride (NaCl) lewis dot structure, polar or nonpolar ... The lewis diagram of an ionic compound is formed with a different approach than the normal procedure we used for drawing the compounds like NH3, BF3, BrF5, etc. Steps to draw electron dot structure or lewis dot structure of NaCl Lewis structures for NH3? - Answers The Lewis dot structure for NH3 starts with an N atom connected on three sides with a dash, each to an H atom. The N atom then has two dots on the unconnected side.
› 49212961 › Inorganic_Chemistry_by(PDF) Inorganic Chemistry by Miessler ~ 5th Edition | Arnab ... This book is ideal for who want to use a strong molecular-orbital approach to explain structure and reactivity in inorganic chemistry. What is the Lewis structure of NH3? | Socratic The Lewis structure of ammonia, #NH_3#, would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons on top of the atom.This is the reason why ammonia acts as a Lewis base, as it can donate those electrons. Dot structure for NH3? - Answers The Lewis dot structure for NH3 starts with an N atom connected on three sides with a dash, each to an H atom. The N atom then has two dots on the unconnected side. What is the Lewis dot diagram for nh3? - FindAnyAnswer.com Also, what is the Lewis dot structure for nh3? Explanation: The Lewis structure of ammonia, NH3 , would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons on top of the atom. This is the reason why ammonia acts as a Lewis base, as it can donate those electrons.
Ammonia (NH3) Lewis Structure - Steps of Drawing NH 3 lewis structure. In the lewis structure of NH 3, there are three N-H bonds and one lone pair on nitrogen atom.There are no lone pairs on hydrogen atoms which cannot keep more than two electrons. Steps of drawing lewis structure of NH 3. You have to follow several steps to draw the lewis structure of NH 3.But, because ammonia is a simple molecule, these steps are not complex and do not ... Lewis Structure for NH3 (Ammonia) - UMD Drawing the Lewis Structure for NH 3 ( Ammmonia) Ammonia (NH 3) is a commonly tested Lewis structure due to it's widespread use in agriculture as a fertilizer. It also is a good example of a molecule with a trigonal prymidal molecular geometry. There are 8 valence electrons available for the Lewis structure for NH 3. NH3 Lewis Structure - How to Draw the Dot Structure for ... A step-by-step explanation of how to draw the NH3 Lewis Dot Structure (Ammonia).For the NH3 structure use the periodic table to find the total number of vale... Nh3 Lewis Structure - lewis dot diagram for nh3 wiring ... Nh3 Lewis Structure - 19 images - represente a estrutura de lewis para o nh3 v rias estruturas, 2 9 chemical bonding benchmark review part ii, lewis structures molecular geometry bond angle and more, ppt covalent bonds powerpoint presentation free,
How to draw NH3 Lewis Structure? - Science Education and ... To sketch the NH3 Lewis structure by following these instructions: Step-1: NH3 Lewis dot Structure by counting valence electrons on the nitrogen atom. Step-2: Lewis Structure of NH3 for constructing around the central nitrogen atom. Step-3: Lewis dot Structure for NH3 generated from step-1 and step-2.
Electron Dot Diagram For Nh3 - schematron.org The Lewis structure for NH3 is.The Lewis dot structure for NH3 starts with an N atom connected on three sides with a dash, each to an H atom. The N atom then has two dots on the unconnected side. NH3, commonly known as ammonia, is arranged as a T-shaped molecule with nitrogen at its center and three hydrogen atoms at its extremities.
NH3 Lewis Structure, Molecular Geometry, Hybridization ... NH3 Lewis Structure, Molecular Geometry, Hybridization, Bond Angle & Shape. Ammonia is a colorless compound, used in making fertilizers. It is a stable hydride formed of one nitrogen and three hydrogen atoms. The molecule has a pungent smell. It can form an NH4+ ion by accepting a proton.
Solved Draw the Lewis dot structure for NH3 and indicate ... Solved Draw the Lewis dot structure for NH3 and indicate the | Chegg.com. Science. Chemistry. Chemistry questions and answers. Draw the Lewis dot structure for NH3 and indicate the shape of the molecule. Lewis dot structure: Shape: Question: Draw the Lewis dot structure for NH3 and indicate the shape of the molecule. Lewis dot structure: Shape:
The Lewis Dot Structure for NH3 - MakeTheBrainHappy The Lewis Dot Structure for NH3. Created by MakeTheBrainHappy. The Lewis Dot Structure for NH3 (Ammonia) is shown above. You could also represent the bonds as dots between the two atoms, but this may be confused with the lone pair electrons on the nitrogen. Each atom in the bond has a full valence shell, with nitrogen having access to eight ...
Lewis Dot Diagram Of Nh3 Lewis Dot Diagram Of Nh3 Lewis Dot Diagram Of Nh3 The Lewis structure of ammonia, NH3, would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons. Electron Dot Structure of NH3 by Jeff Bradbury - February 17, - Lewis Electron Dot Structure for ammonia molecule NH3. by crator-avatar Jeff Bradbury 2.
What is the Lewis dot structure for ammonia? - Colors ... What is the Lewis dot structure for ammonia? In the lewis structure of ammonia (NH3), there are three N-H bonds and one lone pair on nitrogen atom. Lewis structure of NH3 can be drawn by starting from valence electrons of nitrogen and hydrogen atoms in several steps. Can you draw the electron dot structure of […]
PDF Lewis Dot Structures Pogil Key - Hudson City School District Step 4: There were 8 valence electrons in step 1 and 8 electrons in the Lewis dot structure. Application: Use the atom cards and Cheerios to build the molecule before drawing it. 1. 2. 3. Complete the Lewis dot Structure for CC14 Complete the Lewis dot structure for NH3 Complete the Lewis dot structure for H20 -c-CI:
Lewis Structure Of Nh3 - ViralListClub.com Lewis Dot Diagram Of Nh3 The Lewis structure of ammonia NH3 would be three hydrogen atoms bonded to a nitrogen atom in the middle with a lone pair of electrons. Ammonia NH3 or H3N CID 222 - structure chemical names physical and chemical properties classification patents literature biological activities safety.
patapum.to.itFlour Mill Rye [4MH368] Search: Rye Flour Mill. What is Rye Flour Mill. Every flour has its own unique properties. Sourdough Rye using your flour and some crushed organic caraway seeds has lifted my Sourdough Rye to a new level!!
issuu.com › osanoothu › docsDoc 117 b p s xi chemistry iit jee advanced study ... - Issuu Sep 05, 2016 · BRILLIANT PUBLIC SCHOOL, SITAMARHI (Affiliated up to +2 level to C.B.S.E., New Delhi) Class-XI IIT-JEE Advanced Chemistry Study Package Session: 2014-15 Office ...
What is Lewis dot structure of AlCl3? - JanetPanic.com What is the dot structure of NH3? It is a colourless alkaline gas. Starting with the Lewis dot structure of Ammonia, Nitrogen has 5 valence electrons and each hydrogen has 1 valence electron. So, the total valence electrons are 8. Hydrogen always goes on the outside, so Nitrogen is the central atom. What is the structural formula for NH3 ...
Lewis Dot Diagram Of Nh3 - schematron.org What Is the Lewis Dot Structure of NH3? NH3, commonly known as ammonia, is arranged as a T-shaped molecule with nitrogen at its center and three hydrogen atoms at its extremities. Each hydrogen atom is covalently bonded to the nitrogen via an electron pair, and another pair of electrons is attached. Drawing the Lewis Structure for NH 3.
What is the electron dot structure for nh3? The electron-dot structure of NH3 places one pair of nonbonding electrons in the valence shell of the nitrogen atom. This means that there are three bonded atoms and one lone pair for a coordination number of four around the nitrogen, the same as occurs in H2O. The Lewis dot structure for ammonia, NH3. Furthermore, what is the shape of nh3 ...
Lewis Dot Diagram Nh3 - Novocom.top dot diagram nh3 lewis student diagrams which drew shown students nor neither alqurumresort flordelis says . î Lewisî î Dotî î Diagramî For î Nh3î exatin info . lewis nh3 structure ammonia structures trigonal pyramidal nh sundin dimensional molecular three edu forces uwplatt .
How is the Lewis dot diagram for nh3 determined? - Quora What is the Lewis structure of NH4NO3? NH4NO3 is an ionic compound made up of two poly atomic ions, NH4+ and NO3- . NH4+ has 8 valence electrons with the N in the center with sp3 hybridization (tetrahedral) with 4 H atoms bonded to it using the 8 electrons. NO3- has 24 valence electrons with the N in the center and the 3 O atoms bonded to it.

0 Response to "38 lewis dot diagram for nh3"
Post a Comment