42 complete the enthalpy diagram for an ionic compound dissolving in water
CHEM 4311 HW6 *in progress collab CR DONE Flashcards | Quizlet The following solutions are prepared by dissolving the requisite amount of solute in water to obtain the desired concentrations. Rank the solutions according to their respective osmotic pressures in decreasing order assuming the complete dissociation of ionic compounds. Rank from highest to lowest osmotic pressure. Solutions and Their Properties Flashcards | Quizlet It is the energy stored in the intermolecular attractions that hold particles together in an ionic solid. It is the energy of a solute. It is the amount of enthalpy change that occurs when 1mol of ionic solid is converted into gaseous ions. An ionic solid has lattice energy of 6473 kJ/mol and a hydration enthalpy of −6443 kJ/mol.
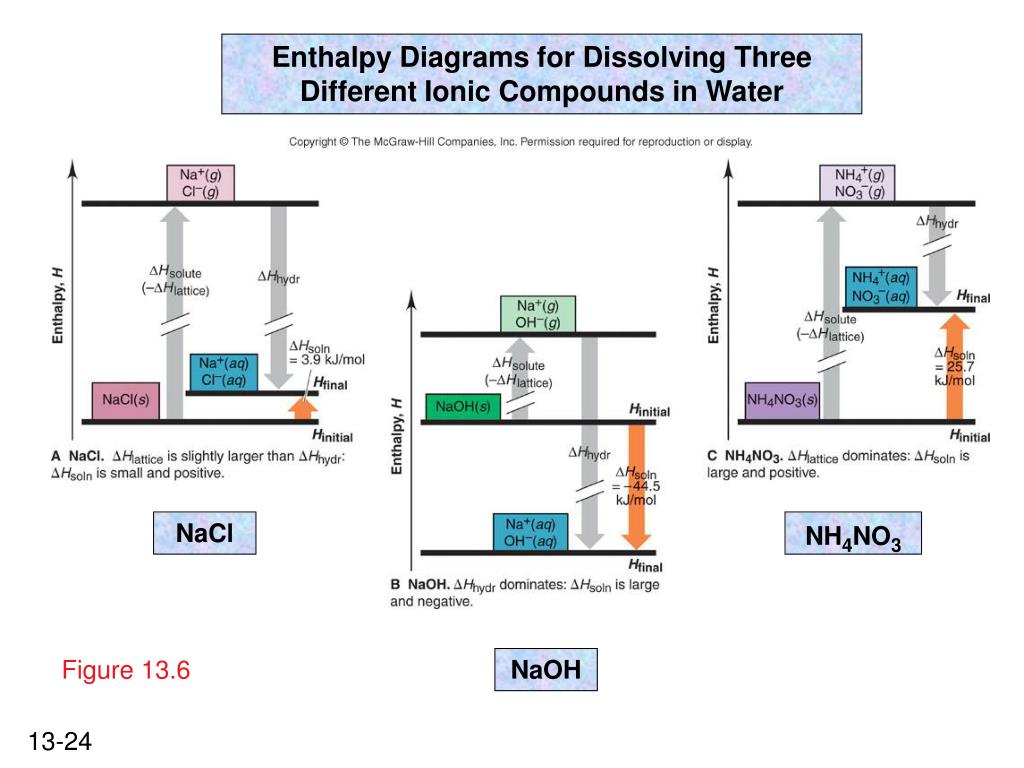
CHAPTER 2.0 THERMOCHEMISTRY_NOTES & TUTORIAL Q's - Flip ... When an ionic compound dissolves in water, positive end of water molecule will attract the negative ion & negative end of water will attract the positive ion DISSOLUTION OF IONIC SOLID INVOLVES 2 STEPS Step 1 Dissociation of lattice : Energy absorbed is the lattice dissociation energy MX(s) H2O M+ (g) + X− (g) Step 2
Complete the enthalpy diagram for an ionic compound dissolving in water
Solved Complete the enthalpy diagram for an ionic compound ... Chemistry. Chemistry questions and answers. Complete the enthalpy diagram for an ionic compound dissolving in water B (g) Y (g) AHsolution B' (aq) Y- (aq) Ahydration Ht final exothermic H solute AHvaporization BY (S) Hinitial endothermic Hormation This reaction is. complete the enthalpy diagram for an ionic compound ... complete the enthalpy diagram for an ionic compound dissolving in water. Answer. + 20. Watch. 1. Why do ionic compounds dissolve in water? | Socratic Ionic compounds dissolve in water because the water molecules hydrate the ions. > To dissolve an ionic compound, the water molecules must be able to stabilize the ions that result from breaking the ionic bond. They do this by hydrating the ions. Water is a polar molecule. It has a permanent dipole. The "O" atom has a partial negative charge, and the "H" atoms have a partial positive charge.
Complete the enthalpy diagram for an ionic compound dissolving in water. PDF FACTFILE: GCE CHEMISTRY - Council for the Curriculum ... Dissolving ionic compounds in water When an ionic compound dissolves in water two processes occur 1. Energy has to be taken in to break up the lattice and separate the positive and negative ions. This is the lattice enthalpy 2. The ions become surrounded by solvent and bonds form - energy is released when these ions form bonds with water molecules. Lab 11 - Thermodynamics of Salt Dissolution Below is an example of a reaction for the dissolution of an ionic compound in water. NaCl ( s) → Na + ( aq) + Cl - ( aq ) When a soluble ionic compound is placed in water, the solid is converted to the product of the dissolution reaction—the solid vanishes, converting to dissolved ions in solution. 3 Complete the enthalpy diagram for an ionic compound ... The diagram represents the solution process (lattice energy, heat of hydration, and heat of solution) for an ionic compound dissolving in water. ht Arrow Brepresents enthalpy of mixing and is exothermic. Arrow Crepresents heat of solution and is exothermic. Arrow Crepresents heat of solution and is endothermic. PDF Thermodynamics of Salt Dissolution - WebAssign Some ionic compounds dissolve readily in water, while others are insoluble. Some ionic com-pounds give o heat when dissolving in water and others absorb heat. Whether the dissolution process of a given ionic compound gives o or absorbs heat depends on the strength of the inter-molecular forces holding the solid together, as well as those ...
Solved Complete the enthalpy diagram for an ionic compound ... Chemistry. Chemistry questions and answers. Complete the enthalpy diagram for an ionic compound dissolving in water. exothermic B* (9) Y- (g) AHvaporization AHsolute B* (aq) Y- (aq) endothermic Enthalpy, H Hfinal Anydration Arusion BY (s) A Hormation Hinitial AH solution This reaction is. Answered: Compound XY is an ionic compound that… | bartleby Compound XY is an ionic compound that dissociates as it dissolves in water. The lattice energy of XY is-591.8 kJ mol. The hydration energy of its ions is -627.8 kJ mol". Write the thermochemical equations for the two steps in the formation of a solution of XY in water. Draw an enthalpy diagram for the formation of this solution. Dissolving Process | Chemistry for Non-Majors The Dissolving Process. Water typically dissolves many ionic compounds and polar molecules. Nonpolar molecules such as those found in grease or oil do not dissolve in water. We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. Water molecules move about continuously due to ... Unit-5-Chemistry-questions.doc - Name Unit 5 Chemistry ... Sodium chloride is an ionic compound that dissolves in water. The solution contains aqueous ions Na + (aq) and Cl - (aq). A concentrated aqueous solution of sodium chloride is known as brine. (a) Standard enthalpy changes of hydration can be used as part of an energy cycle to predict the solubility of an ionic compound, such as sodium ...
What dissolves in salt ionic? - JacAnswers Thus you can break the ionic bonds just by dissolving the ionic compound in water. Is li3p ionic or covalent? In Li3P, lithium phosphide there is Li+ cation and P-3as anion ,so definitely this compond is the ionic compound. What is complete ionic equation? Complete ionic equation: a molecular equation that separates the molecules into their ion ... Chemistry Chapter 7 Flashcards - Quizlet Terms in this set (70) Combustion Reactions. A reaction in which a substance reacts with oxygen, emitting heat and forming one or more oxygen-containing compounds. Evidence of a chemical reaction: -color change. -formation of a solid in a previously clear solution. -the formation of a gas when we add a substance to a solution. Solved Complete the enthalpy diagram for an ionic compound ... Answer : The given is the complete enthalpy diagram for the ionic compound. The AX (s) is separated into its ion in gaseous forms that is A+ (g) and X- (g). This will require the solute. When the …. View the full answer. Transcribed image text: Complete the enthalpy diagram for an ionic compound dissolving in water. Solved Label the phase diagram of pure solvent and a ... We review their content and use your feedback to keep the quality high. 100% (10 ratings) Transcribed image text: Label the phase diagram of pure solvent and a solution Freezing point of solution GAS Solution Pure solvent 1 atm Boiling pointFreezing point of of solvent solvent ??? 11 SOLID AT AP Boiling pointLIQUID of solution Temperature.
Solved Complete the enthalpy diagram for an ionic compound ... Complete the enthalpy diagram for an ionic compound dissolving in water. A (g) X-(g) AHnydration AHusion exothermic AX(s) initial ??,aporization endothermic A (aq) x-(aq) A Hsolution Hrinal ??,ormation AHsolute This reaction is ; Question: Complete the enthalpy diagram for an ionic compound dissolving in water. A (g) X-(g) AHnydration AHusion ...
PDF Cambridge International Examinations Cambridge ... 1 (a) The dissolving of an ionic compound in water is accompanied by an energy change, the enthalpy change of solution, ∆H sol. MgCl 2+2(s) + aq → Mg (aq) + 2Cl -(aq) Describe, in terms of bond breaking and bond making, what happens to the solid ionic lattice when an ionic compound dissolves in water.
PDF (3) CE = 0 if O2− or water ionic or H bonding 1 (f) Magnesium oxide reacts with water / forms Mg(OH) 2 Allow MgO does not dissolve in water / sparingly soluble / insoluble 1 [11] Q3. (a) Enthalpy change for the formation of 1 mol of gaseous atoms allow heat energy change for enthalpy change 1 From the element (in its standard state)
How does sodium chloride (NaCl) dissolve in water? Sodium chloride (NaCl) dissolves when water molecules continuously attack the NaCl crystal, pulling away the individual sodium (Na +) and chloride (Cl -) ions. This nonstop attack continuous until the whole NaCl crystal disintegrates. To understand this process at the molecular level, we must apply the three steps we previously discussed.
PDF Lattice Enthalpy NEW - WordPress.com Enthalpy change of solution and Enthalpy change of Hydration When ionic compounds dissolve in water, there is usually a temperature change. Sometimes this is exothermic (e.g. dissolving calcium chloride) and sometimes endothermic (e.g. dissolving ammonium nitrate). The experiments are easy to carry out in a laboratory, and the usual equations ...
Solubility - Purdue University We can generally assume that salts dissociate into their ions when they dissolve in water. Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic bonds in the solid and the energy required to separate the water molecules so that the ions can be inserted into solution.
Chemistry 1 Exam Flashcards - Quizlet Calculate the amount of heat required (in kilojoules) to heat 5.00 grams of water from -16.0 C to 11.0 c.-enthalpy of vaporization for water is 40.56 kJ/mol-enthalpy for fusion of water is 6.007 kJ/mol -specific heat for ice is 2.090 J/(gram x *C)-specific heat for water is 4.184 J/(gram x *C)-specific heat for steam is 2.030 J/(gram x *C)
Water molecules and their interaction with salt | U.S ... Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a homogeneous solution.
Why do ionic compounds dissolve in water? | Socratic Ionic compounds dissolve in water because the water molecules hydrate the ions. > To dissolve an ionic compound, the water molecules must be able to stabilize the ions that result from breaking the ionic bond. They do this by hydrating the ions. Water is a polar molecule. It has a permanent dipole. The "O" atom has a partial negative charge, and the "H" atoms have a partial positive charge.
complete the enthalpy diagram for an ionic compound ... complete the enthalpy diagram for an ionic compound dissolving in water. Answer. + 20. Watch. 1.
Solved Complete the enthalpy diagram for an ionic compound ... Chemistry. Chemistry questions and answers. Complete the enthalpy diagram for an ionic compound dissolving in water B (g) Y (g) AHsolution B' (aq) Y- (aq) Ahydration Ht final exothermic H solute AHvaporization BY (S) Hinitial endothermic Hormation This reaction is.

0 Response to "42 complete the enthalpy diagram for an ionic compound dissolving in water"
Post a Comment