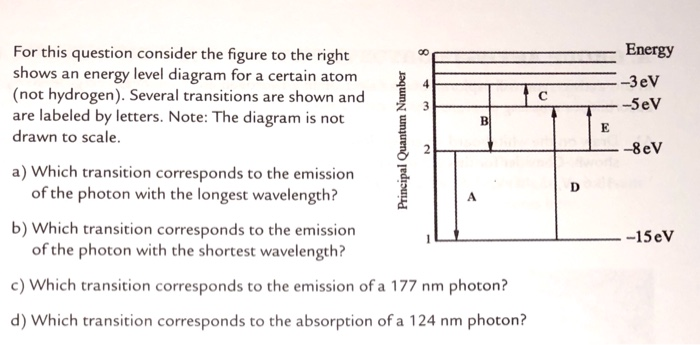
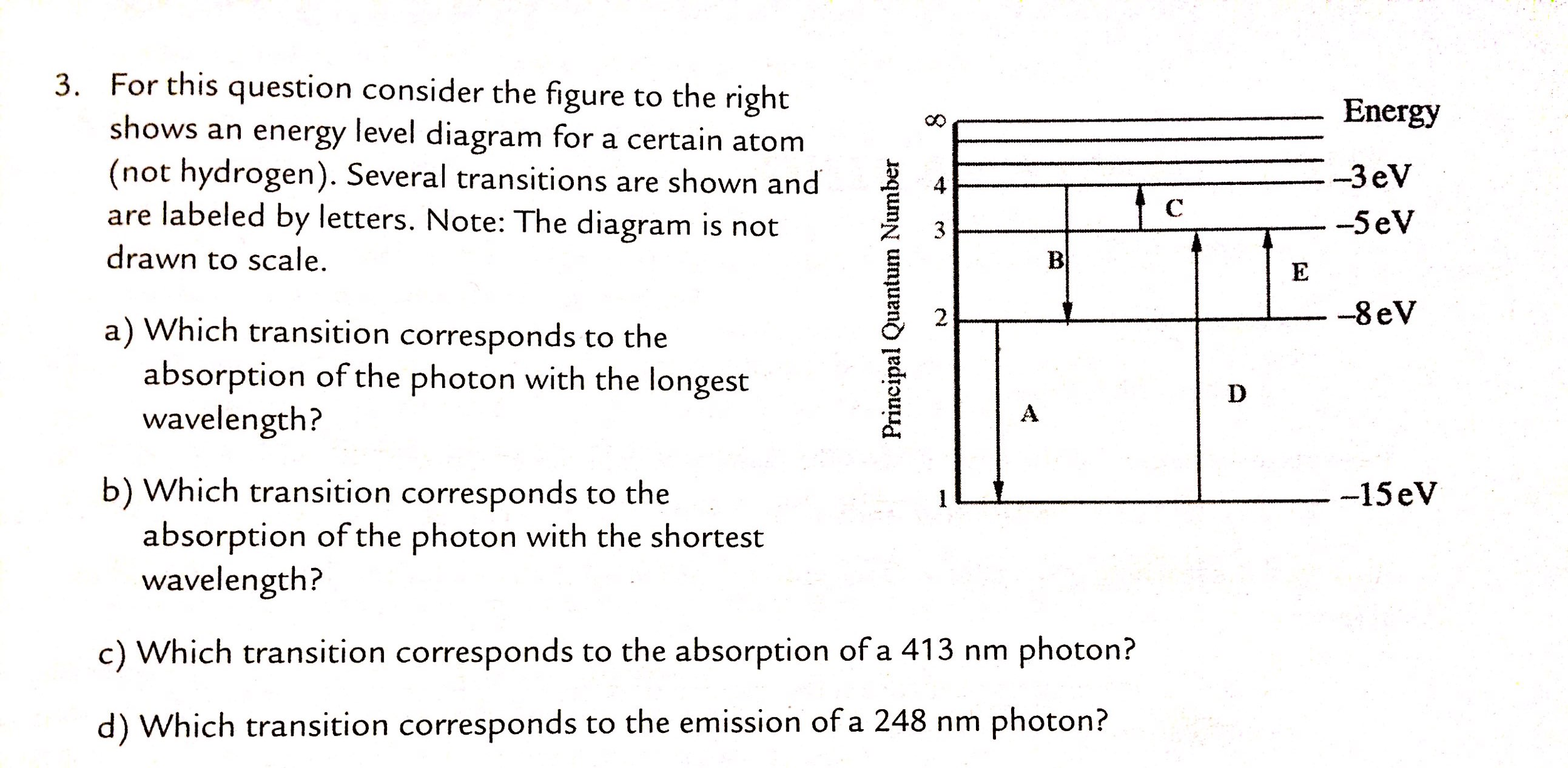
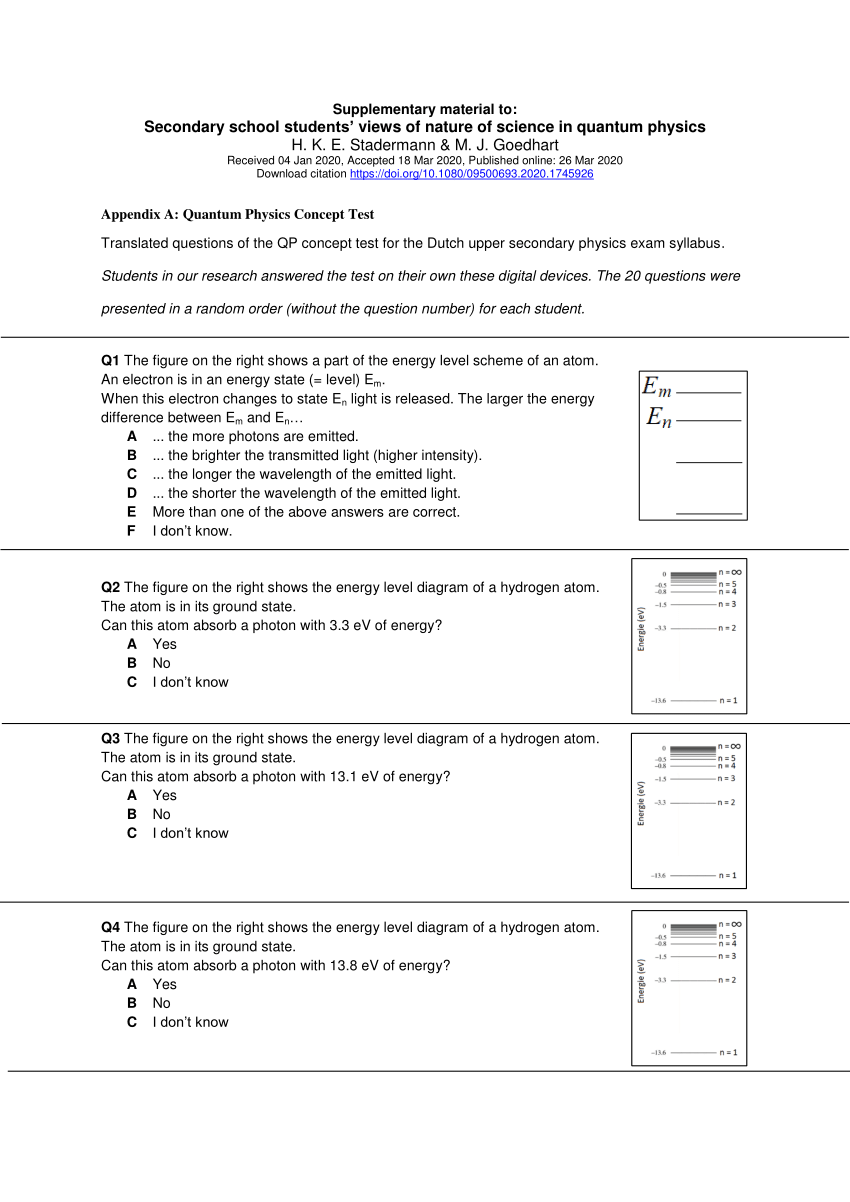
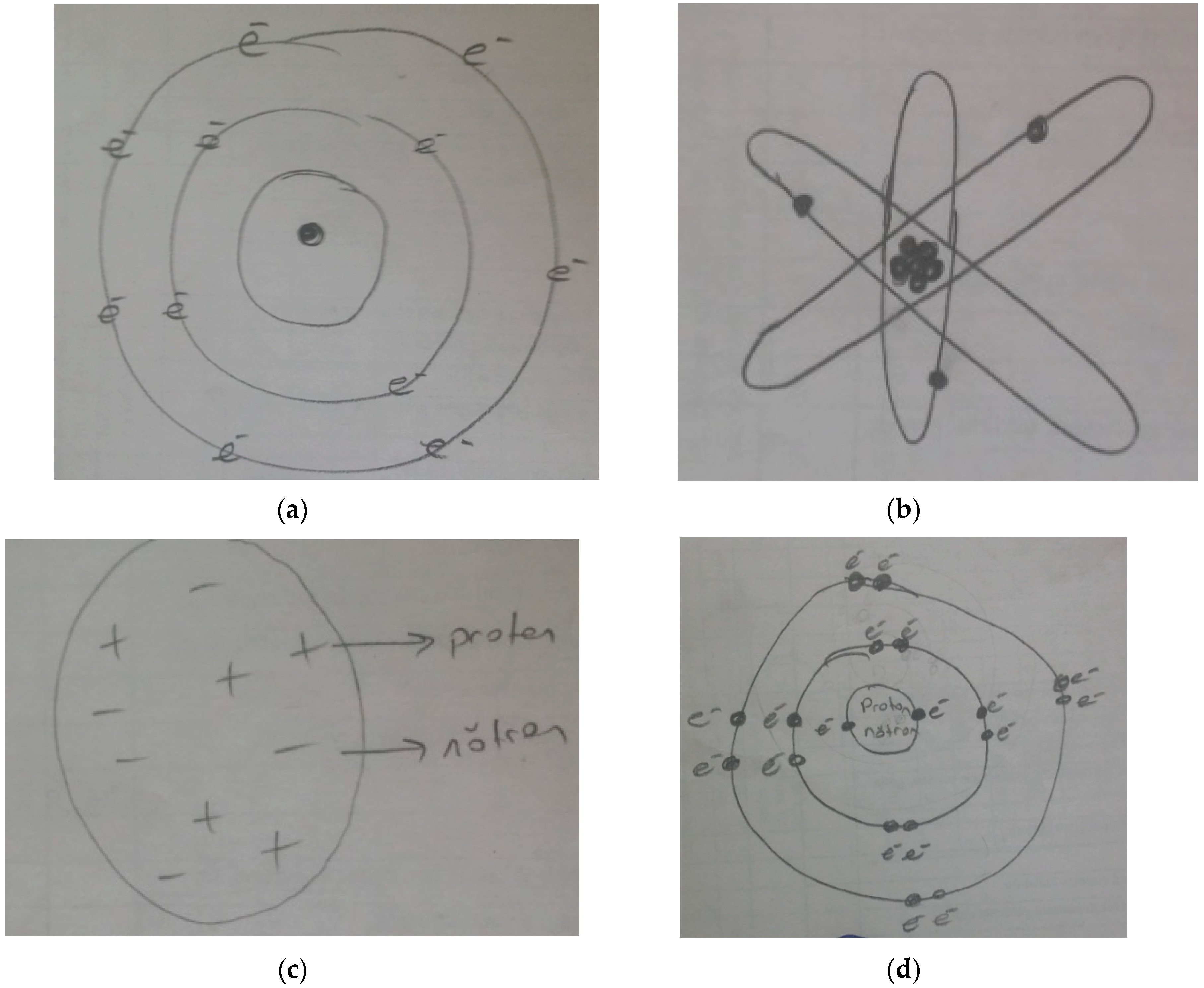
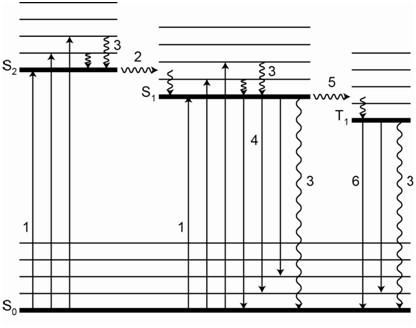
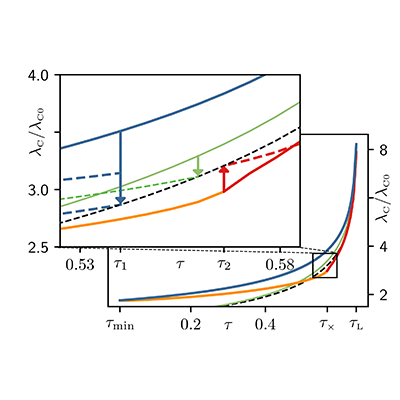
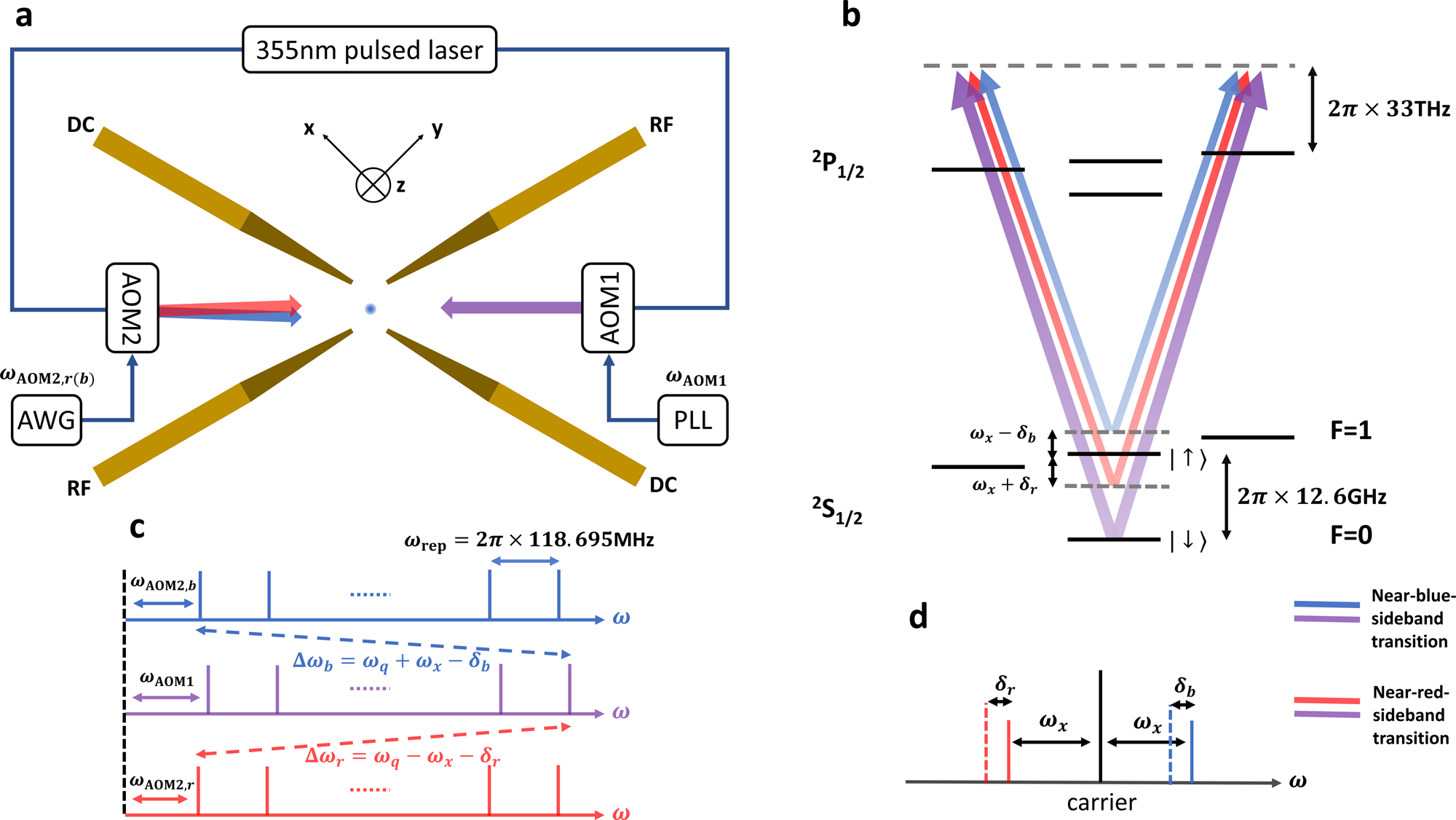
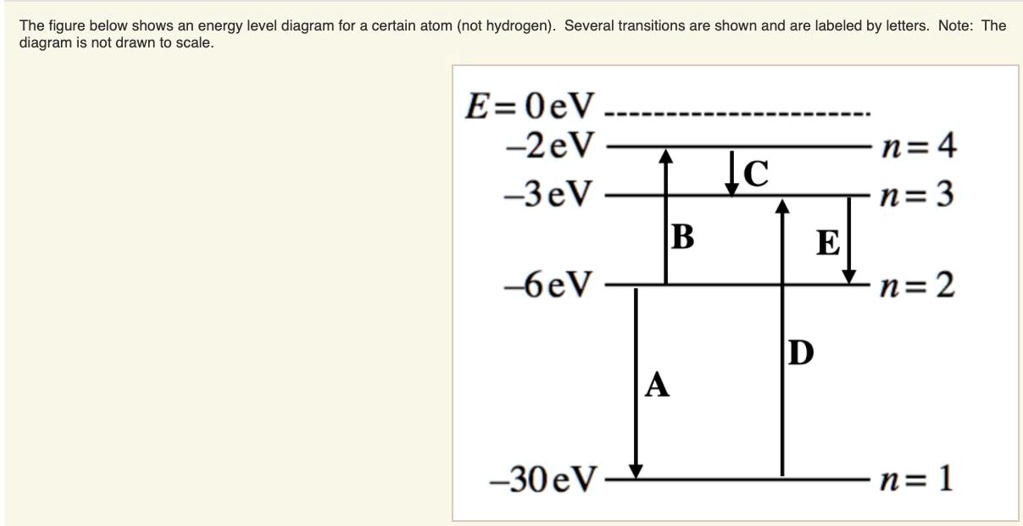
42 for this question consider the figure to the right shows an energy level diagram for a certain atom
The diagram shows the energy levels for an electron ... - Toppr The energy diagram is drawn with appropriate scale to indicate difference in energy levels. The largest energy difference between states is between 1 and 2.1 answer · Top answer: Step 1 - Drawing the figure and mentioning all the value. Step 2 - Obtaining the answer, by using the transition of photon concept. in I : Energy ... Answered: 2. An electron in an energy level of an… | bartleby Science Advanced Physics Q&A Library 2. An electron in an energy level of an atom moves in a circular path around a nucleus. The radius of the path is 1.2 x 1011m and the speed of the electron is 1.03 x 107 ms-1 (a) Find the centripetal acceleration of the electron.
Energy Level Diagram For Hydrogen - Mini Physics The ionization energy of an atom is the energy required to remove the electron completely from the atom. (transition from ground state n = 0 to infinity n = ∞ ). For hydrogen, the ionization energy = 13.6eV. When an excited electron returns to a lower level, it loses an exact amount of energy by emitting a photon.

For this question consider the figure to the right shows an energy level diagram for a certain atom
quizlet.com › 186646475 › chem-180-set-2-flash-cardsChem 180 set 2 Flashcards - Quizlet Look at the phase diagram of H2O in Figure 10.28 in the textbook, and tell what happens to an H2O sample when the following changes are made. The temperature is reduced from 48∘C to -4.4∘C at a constant pressure of 6.5 atm. The pressure is increased from 85 atm to 226atm at a constant temperature of 380∘C. Solved For this question consider the figure to the right ... Physics. Physics questions and answers. For this question consider the figure to the right shows an energy level diagram for a certain atom (not hydrogen). Several transitions are shown and are labeled by letters. Which transition corresponds to the emission of the photon with the longest wavelength? PDF 4. Energy Levels - MIT OpenCourseWare 4.3.1 The Hydrogen atom In the previous chapter we studied stationary problems in which the system is best described as a (time-independent) wave, "scattering" and "tunneling" (that is, showing variation on its intensity) because of obstacles given by changes
For this question consider the figure to the right shows an energy level diagram for a certain atom. Potential Energy Diagrams - Kentchemistry.com A potential energy diagram plots the change in potential energy that occurs during a chemical reaction. This first video takes you through all the basic parts of the PE diagram. Sometimes a teacher finds it necessary to ask questions about PE diagrams that involve actual Potential Energy values. Solved For this question consider the figure to the right ... Science Physics Physics questions and answers For this question consider the figure to the right shows an energy level diagram for a certain atom (not hydrogen). Several transitions are shown and are labeled by letters. Note: The diagram is not drawn to scale. Solution for Student Worksheet: Energy Levels in the Atom Solution for Calculate the Energy! Student Worksheet Neils Bohr numbered the energy levels (n) of hydrogen, with level 1 (n=1) being the ground state, level 2 being the first excited state, and so on.Remember that there is a maximum energy that each electron can have and still be part of its atom. Beyond that energy, the electron is no longer bound to the nucleus of the atom and it is ... Which Energy Level Transition Results in the Highest ... A hydrogen atom becomes ionized when its electrons have enough energy to jump out of the outermost energy level and become free of the atom. An electron near to escaping the atom has the most energy and therefore emits a high-energy photon if it drops all the way to level one. If the photon contains more energy, it has a higher frequency.
Solved For this question consider the figure to the right ... Question: For this question consider the figure to the right shows an energy level diagram for a certain atom (not hydrogen). Several transitions are shown and are labeled by letters. Note: The diagram is not drawn to scale. Which transition corresponds to the emission of the photon with the longest wavelength? PDF Waves review practice questions - The Leon M. Goldstein ... 46. The diagram shows two pulses, Aand B, moving to the right along a uniform rope. Compared to pulse A, pulse Bhas A. a slower speed and more energy B. a faster speed and less energy C. a faster speed and the same energy D. the same speed and more energy 47. A wave generator located 4.0 meters from a re ecting wall produces a standing wave in a PDF ANSWER. Series #2 - LSU Figure 2 Refer to Figure 2 when answering questions #10 and #11. 10. In the two-level atom shown in Figure 2, which electron transition is associated with the emission of light? ANSWER. The transition labeled "b". 11. If the "c" transition marked in the three-level atom is associated with the absorption or The figure is an energy-level diagram for a simple atom. For this question consider the figure to the right that shows an energy level diagram for a certain atom (not hydrogen). Several transitions are shown and labeled by letters. Note the diagram is not drawn to scale. (A) Which transition corresponds to the emission of the photon with the longest wavelength?
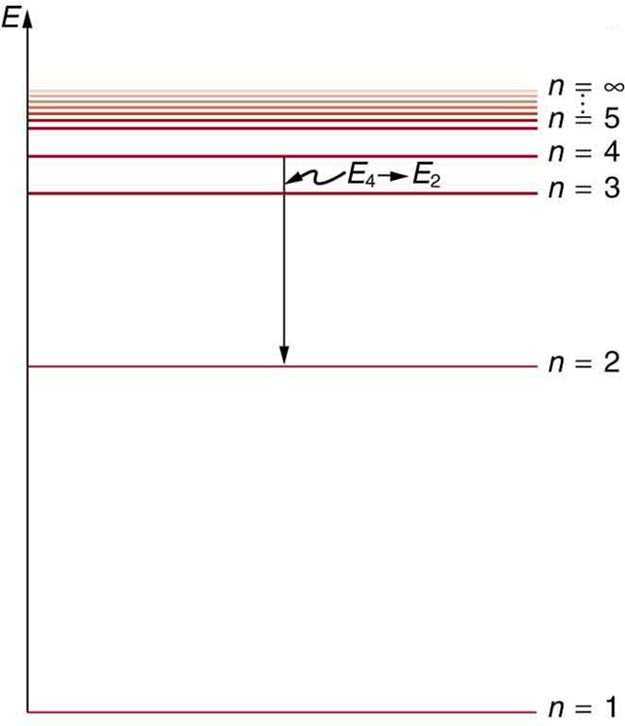
› classzone-retiredClasszone.com has been retired - Houghton Mifflin Harcourt Social-Emotional Learning Curriculum. Research shows that a social-emotional learning curriculum can lead to improved academic performance. Explore high-quality SEL programs that get results. Bohr's Theory of the Hydrogen Atom | Physics Figure 1. Niels Bohr, Danish physicist, used the planetary model of the atom to explain the atomic spectrum and size of the hydrogen atom. His many contributions to the development of atomic physics and quantum mechanics, his personal influence on many students and colleagues, and his personal integrity, especially in the face of Nazi oppression, earned him a prominent place in history. Chapter 5 Concept Quiz Flashcards | Quizlet B. You can tell because the electron jumps up from 0 eV to 10.2 eV. The diagram represents energy levels in a hydrogen atom. The labeled transitions (A through E) represent an electron moving between energy levels. Suppose that an electron in a hydrogen atom absorbs 10.2 eV of energy, so that it moves from level 1 to level 2. 22.1 The Structure of the Atom - Physics | OpenStax Figure 22.10 An energy-level diagram plots energy vertically and is useful in visualizing the energy states of a system and the transitions between them. This diagram is for the hydrogen-atom electrons, showing a transition between two orbits having energies E 4 E 4 and E 2 E 2. The energy transition results in a Balmer series line in an ...
Answered: 9. The figure to the right shows an… | bartleby The figure to the right shows an energy Energy level diagram for a certain atom (not hydrogen). Several transitions are shown and are labeled by letters.1 answer · Top answer: Introduction “Since there are multiple questions and it is not mentioned that which one has to be solved so I am solving only the first question. ...
› topic › Physics1085 questions with answers in PHYSICS | Science topic Feb 18, 2013 · In figure 2, it demonstrates how electrolytes affect the structure of the polymer; in figure 3, it shows how at a state of a helical rod and no longer a random coil, it is capable to hydrogen ...
byjus.com › wbjee › wbjee-2020-physics-question-paperWBJEE 2020 Physics Solved Question Paper - Download PDF Question 26: The frequency v of the radiation emitted by an atom when an electron jumps from one orbit to another is given by v = kδE, where k is a constant and δE is the change in energy level due to the transition. Then dimension of k is
Background: Atoms and Light Energy - NASA The ground state of an electron, the energy level it normally occupies, is the state of lowest energy for that electron. There is also a maximum energy that each electron can have and still be part of its atom. Beyond that energy, the electron is no longer bound to the nucleus of the atom and it is considered to be ionized.
Principles of Physics: A Calculus-Based Text Raymond A. Serway, John W. Jewett · 2012 · EducationDue to electronic rights, some third party content may be suppressed from the eBook ... Figure P29.6 An energy level diagram for hydrogen showing the Balmer ...
OneClass: The figure is an energy-level diagram for a ... For this question consider the figure to the right that shows an energy level diagram for a certain atom (not hydrogen). Several transitions are shown and labeled by letters. Note the diagram is not drawn to scale. (A) Which transition corresponds to the emission of the photon with the longest wavelength?
Energy Level Diagram - Different Energy Shells Around the ... What is energy level diagram? In chemistry, an electron shell, or energy level, may be imagined as an orbit with electrons around the nucleus of an atom. The closest shell to the nucleus is called the "K shell" followed by the "L shell" then the "M shell" and so on away from the nucleus.
Atomic Energy Levels (video) - Khan Academy We like representing these energy levels with an energy level diagram. The energy level diagram gives us a way to show what energy the electron has without having to draw an atom with a bunch of circles all the time. Let's say our pretend atom has electron energy levels of zero eV, four eV, six eV, and seven eV.
SOLVED:For this question consider the figure to the right shows an ... For this question consider the figure to the right shows an energy level diagram for certain atom (not hydrogen). Several transitions are shown and are ...4 answers · Top answer: In this exercise, we have the bomber Siri's, which comes. It consists off transitions ...
› I_03The Feynman Lectures on Physics Vol. I Ch. 3: The Relation of ... Consider this analogy: If we wanted to take an object from one place to another, at the same level but on the other side of a hill, we could push it over the top, but to do so requires the addition of some energy. Thus most chemical reactions do not occur, because there is what is called an activation energy in the way.
For this question consider the figure that... | Clutch Prep Problem: For this question consider the figure that shows an energy level diagram for a certain atom (not hydrogen).Several transitions are shown and are labeled by letters. Note: The diagram is not drawn to scale. Which transition corresponds to the emission of the photon with the longest wavelength?
Energy Level and Transition of Electrons | Brilliant Math ... In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. According to Bohr's theory, electrons of an atom revolve around the nucleus on certain orbits, or electron shells. Each orbit has its specific energy level, which is expressed as a negative value. This is because the electrons on the orbit are "captured ...
Molecular Symmetry - Page 142 - Google Books Result David Willock · 2009 · Science... z 2 , x 2 –y 2 Δ t Z X Y Figure 5.18 The d-orbital energy levels for the central atom of a Td complex; the diagram to the right shows the reference axis ...
The Structure of the Atom | Astronomy - Lumen Learning To take a specific example, consider a calcium atom inside the Sun's atmosphere in which an electron jumps from a lower level to a higher level. To do this, it needs about 5 × 10 -19 joules of energy, which it can conveniently obtain by absorbing a passing photon of that energy coming from deeper inside the Sun.
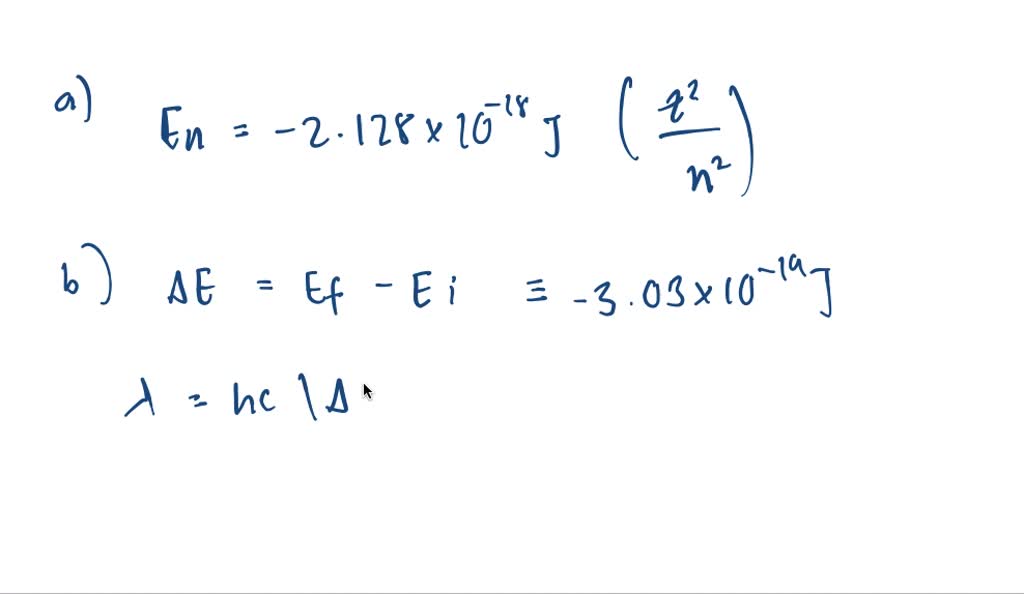
the figure below shows an energy level diagram for certain atom not hydrogen several transitions are shown and are labeled by letters_ note the diagram is not drawn t0 scale_ e oev zev s3ev 51005
Energy Level of an Atom - Energy State and ... - VEDANTU The figure shows the energy levels of an atom. The first four energy levels are shown here. The first energy level is also called level 'K'. The second level is called level L, third energy level as M, and so on. The electrons from energy level K contains the least energy whereas the levels that are far from the nucleus contains more energy
physics 28-31 Flashcards - Quizlet The figure shows an energy level diagram for the hydrogen atom. Several transitions are shown and are labeled by letters.Note: The diagram is not drawn to scale. Which transition corresponds to the absorption of the photon with the longest wavelength?
CH. 27 Flashcards - Quizlet The figure shows part of the energy level diagram of a certain atom. The energy spacing between levels 1 and 2 is twice that between 2 and 3. If an electron makes a transition from level 3 to level 2, the radiation of wavelength λ is emitted. What possible radiation wavelengths might be produced by other transitions between the three energy ...
› JEvzOlliuqNobrac › science-10Science 10 Learner's Material Unit 2 - SlideShare Jun 20, 2015 · 98 3. Hold the closed test tube horizontally. Shake or roll gently with your fingers to level out the iron filings inside. Figure 7a. Leveling the Iron Filings inside the Test Tube 4. Then when levelled, touch with the north-pole end of the permanent magnet the test tube’s curved end.
Solved ** PLEASE NOTE THIS QUESTION IS DIFFERENT FROM ... For this question consider the figure to the right shows an energy level diagram for a certain atom, (not hydrogen). Several transitions are shown and are labeled by letters. Note: The diagram is not drawn to scale .
Energy level diagrams and the hydrogen atom Energy level diagrams and the hydrogen atom. It's often helpful to draw a diagram showing the energy levels for the particular element you're interested in. The diagram for hydrogen is shown above. The n = 1 state is known as the ground state, while higher n states are known as excited states.
› blogBlogs - ZDNet ZDNet's technology experts deliver the best tech news and analysis on the latest issues and events in IT for business technology professionals, IT managers and tech-savvy business people.
PDF 4. Energy Levels - MIT OpenCourseWare 4.3.1 The Hydrogen atom In the previous chapter we studied stationary problems in which the system is best described as a (time-independent) wave, "scattering" and "tunneling" (that is, showing variation on its intensity) because of obstacles given by changes
Solved For this question consider the figure to the right ... Physics. Physics questions and answers. For this question consider the figure to the right shows an energy level diagram for a certain atom (not hydrogen). Several transitions are shown and are labeled by letters. Which transition corresponds to the emission of the photon with the longest wavelength?
quizlet.com › 186646475 › chem-180-set-2-flash-cardsChem 180 set 2 Flashcards - Quizlet Look at the phase diagram of H2O in Figure 10.28 in the textbook, and tell what happens to an H2O sample when the following changes are made. The temperature is reduced from 48∘C to -4.4∘C at a constant pressure of 6.5 atm. The pressure is increased from 85 atm to 226atm at a constant temperature of 380∘C.




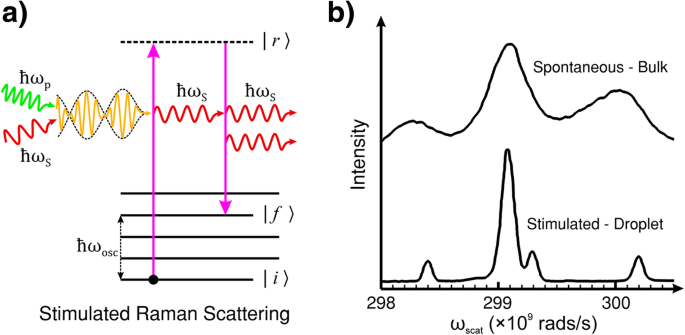





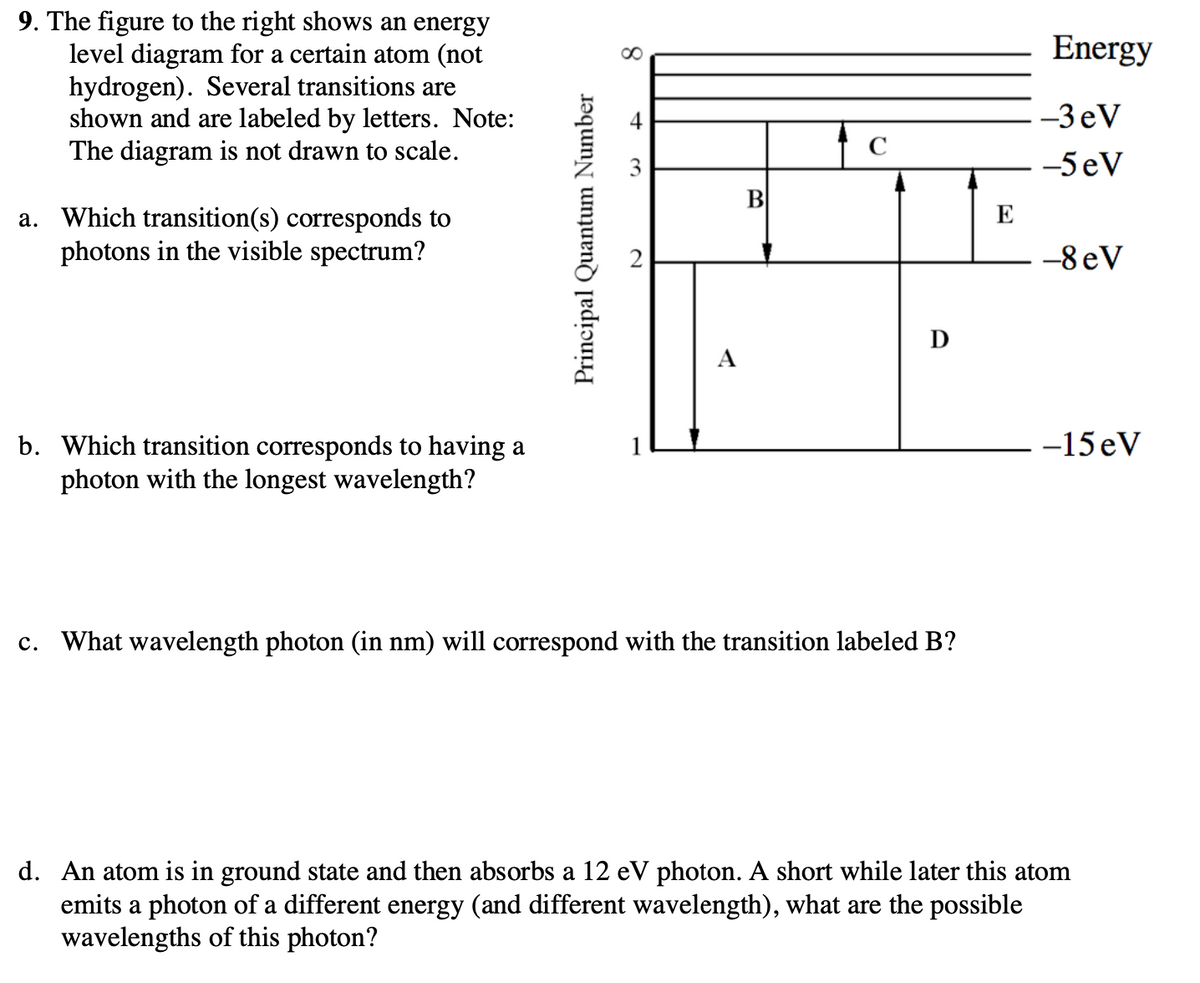












0 Response to "42 for this question consider the figure to the right shows an energy level diagram for a certain atom"
Post a Comment