41 fluorine electron dot diagram
Fluorine (F2) Molecule Lewis Structure Fluorine is a group VIIA element in the periodic table and contains seven electrons in its last shell. Now, we know how many electrons are there in valence shells of fluorine atoms. valence electrons given by fluorine atoms = 7 * 2 = 14 Total valence electrons = 14 Total valence electrons pairs How to draw MgF2 Lewis Structure? - Science Education and Tutorials Step-1: MgF2 Lewis Structure. To calculate the valence electron of each atom in MgF2, look for its periodic group from the periodic table. The alkaline earth metal and halogen families, which are the second and 17th groups in the periodic table, are both made up of magnesium and fluorine atoms. In their outermost shells, magnesium and fluorine ...
Electron Dot Diagram For Fluorine - schematron.org Pictorial Electron dot structure - valence electrons are represented by dots placed around the. Draw a Lewis electron dot diagram for an atom or a monatomic ion. In almost all Fluorine and neon have seven and eight dots, respectively: Fluoride-Neon. A larger outer circle has one red dot on, representing the second shell with one electron.

Fluorine electron dot diagram
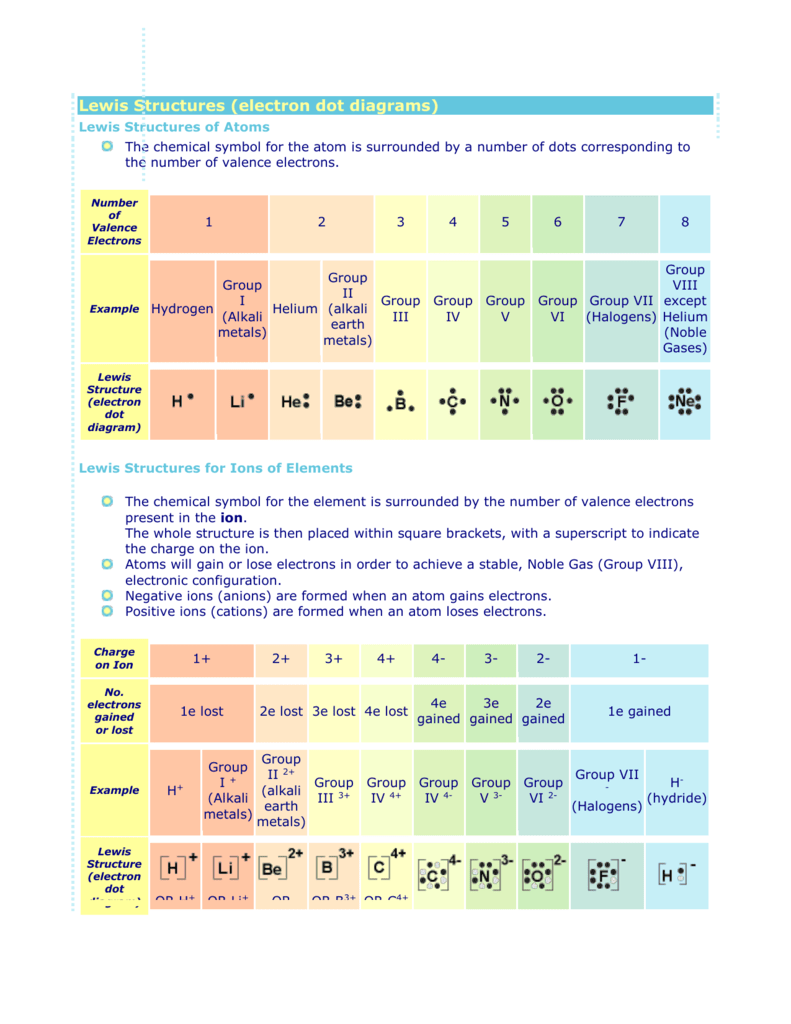
Fluorine Bohr Model - How to draw Bohr diagram for Fluorine(F) atom Electron dot diagram of Fluorine atom Electron dot diagram also called lewis structure which represents the valence electrons of atoms. As, from the Bohr diagram of Fluorine, we got to know, it has 7 valence electrons. So, just represent these 7 valence electrons around the Fluorine atom as a dot. The electron configuration of Fluorine Lewis Dot Diagram For Fluorine Upon reduction, the fluorine atom forms fluoride, which has 8 valence electrons, and is isoelectronic with a Noble Gas (which one?). A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. Electron Dot Diagrams | Chemistry for Non-Majors Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element's symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are ...
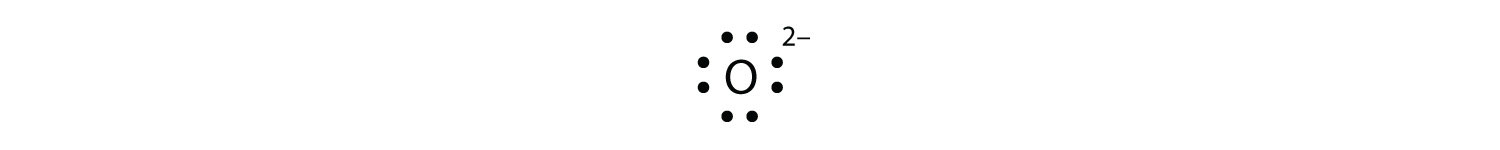
Fluorine electron dot diagram. Ions, Electron Dot Diagrams, Ionic Bonding and Naming Ionic Compounds - Student Note ... Ions, Electron Dot Diagrams, Ionic Bonding and Naming Ionic Compounds When an atom loses or gains electrons to have a full valence shell, it's most stable structure, it will have a charge because of the imbalance of the number of positive protons and negative electrons.This structure that has a charge is called an ion. Above is lithium is illustrated. On the right is the neutral atom of ... Electron Dot Diagram For Fluorine - Wiring Diagrams Electron Dot Diagram For Fluorine A larger outer circle has one red dot on, representing the second shell with one electron. Lithium is in Group 1 of the Periodic Table. Fluorine. Structure of a. Upon reduction, the fluorine atom forms fluoride, which has 8 valence electrons, and is isoelectronic with a Noble Gas (which one?). BeF2 lewis structure, Molecular geometry, Polar or nonpolar, Bond angle Beryllium fluoride (BeF2) lewis dot structure, molecular geometry, electron geometry, polar or nonpolar, bond angle. Beryllium fluoride is an inorganic compound that appears as colorless lumps have a chemical formula BeF2. It is an odorless white solid also known as fluoride salt of beryllium. It is commonly used in biochemistry. What is the Lewis electron-dot diagram for a fluoride ion? - Socratic Fluorine is in Group 17 of the Periodic Table..... And thus the neutral atom has 7 valence electrons. Of course the elemental form is bimolecular. Upon reduction, the fluorine atom forms fluoride, which has 8 valence electrons, and is isoelectronic with a Noble Gas (which one?). Which do you think would be bigger; fluorine atom or fluoride ion?
PDF Atomic Protons Neutrons Electrons Lewis Dot Mass Lewis Dot Diagram Worksheet Use the Bohr models to determine the number of valance electrons. Once you have found the number of valance electrons, place them around the elements symbol. Element Atomic # Atomic Mass Protons Neutrons Electrons Lewis Dot Carbon 6 12 6 6 6 l Hydrogen 1 1 1 0 1 H Lithium 3 7 3 4 3 Li Magnesium Fluoride Lewis Dot Diagram Using Lewis dot diagrams, show how some number of atoms of magnesium and atoms of fluorine can transfer electrons to form ions of each element with stable.What is the correct lewis electron-dot structure for the compound magnesium fluoride? Chemistry Covalent Bonds Drawing Lewis Structures. Electron Configuration for Fluorine (F) - UMD Fluorine is the ninth element with a total of 9 electrons. In writing the electron configuration for fluorine the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for F go in the 2s orbital. The remaining five electrons will go in the 2p orbital. Therefore the F electron configuration will ... How to draw HF Lewis Structure? - Science Education and Tutorials HF Lewis dot structure. To calculate the formal charge on the central fluorine atom of the HF molecule by using the following formula: The formal charge on the fluorine atom of HF molecule= (V. E(F)- L.E(F) - 1/2(B.E)) V.E (F) = Valence electron in a fluorine atom of HF molecule
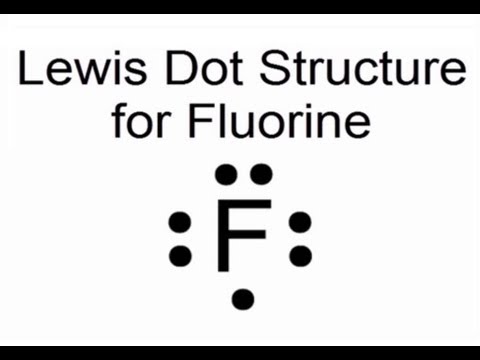
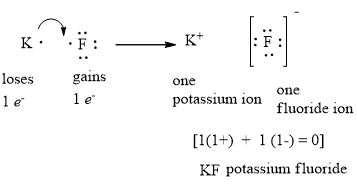
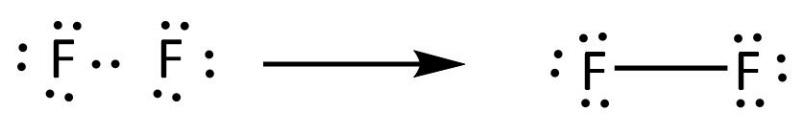
Is SiF4 Polar or Nonpolar: Why, How, Lewis structure, Detailed Explanations The drawing process of electron dot structure of the compound depends on the valance possessed by the elements. Valance of Silicon is four and fluorine is seven. Therefore, the total number of valance electron in SiF4 is [4+ (7*4)] = 32. However, by arranging these valance electrons the elements fulfil their octets. What is the Lewis dot structure for fluorine? - Answers What does the Lewis dot structure of f2 look like? Fluorine has 7 valence electrons each, so they will share one electron. Will look like this, F-F. If your doing a dot structure, just put a pair ... Lewis Dot Structure for Fluorine Atom (F) - YouTube A step-by-step explanation of how to draw the Lewis dot structure for F (Fluorine). I show you where Fluorine is on the periodic table and how to determine ... Lewis Electron Dot Diagram For Fluoride Ion In almost all Fluorine and neon have seven and eight dots, respectively: Fluoride-Neon.The Lewis Structure (electron dot diagram) of each ion is used to construct the Lewis Structure (electron dot diagram) for the ionic compound. Examples. Lithium fluoride, LiF. Lithium atom loses one electron to form the cation Li+.
%Fluorine Lewis Dot Structure:Drawing,Several Compounds And Detailed Explanationstitle ... To draw fluorine lewis dot structure, we have to count valence electrons of Fluorine that is 7 which are written as dots around "F" . So Fluorine needs one electron to fill the Octet structure. It accept electron from donor atom to gain noble gas like stability. Fluorine ion lewis dot structure Fluorine is 'group 17' element in Periodic table.
[Expert Answer] draw the electron dot structure of F2 - Brainly Fluorine is the 9th element of the periodic table having electronic configuration of . Number of valence electrons = (2 + 5) = 7. Fluorine needs 1 electron to complete its octet. When another fluorine combines, they share 1 electron each forming a single bond. Hence, the Lewis dot structure of fluorine molecule is given below.
Drawing dot- and- cross diagrams of Covalent Molecules - O Level - Emily Learning The dot-and- cross diagram of bromine will look like that of fluorine and chlorine. Only that bromine should be the biggest, followed by chlorine and then chlorine. Dot- and- cross diagram of covalent molecule (oxygen) O2 Oxygen is in Group VI of the periodic table. This means that an atom of oxygen has 6 valence electrons.
Diagram - Fluorine Fluorine's Diagram. ... Mass Number: 18.9984032 Electron configuration: 1s2,2s2,2p5 or [HE] 2s2 2p5 Valence Electrons: 7 Orbital Notation: Electron Dot Notation: Powered by Create your own unique website with customizable templates. Get Started ...
Fluorine(F) electron configuration and orbital diagram Fluorine (F) orbital diagram 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital. According to Hund's principle, the first electron will enter in the clockwise direction and the next electron will enter the 1s orbital in the anti-clockwise direction.
Lewis Dot Diagram For Fluorine - schematron.org In Lewis diagrams the atoms are shown by writing the atomic symbol surrounded by one dot for each of the valence electrons. In a covalently bound molecule the dots are arranged in pairs, with the bound pairs placed between the . Fluorine is in Group 17 of the Periodic Table.. And thus the neutral atom has 7 valence electrons.
Fluorine | F2 - PubChem Fluorides, hydrogen fluoride, and fluorine are chemically related. Fluorine is a naturally-occurring, pale yellow-green gas with a sharp odor. It combines with metals to make fluorides such as sodium fluoride and calcium fluoride, both white solids. Sodium fluoride dissolves easily in water, but calcium fluoride does not. Fluorine also combines with hydrogen to make hydrogen fluoride, a ...
6.1 Lewis Electron Dot Diagrams | Introductory Chemistry A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ...
Which is the correct Lewis structure for fluorine, which is a group 7A element ... Explanation : Lewis-dot structure : It is defined as the representation which shows the valence electrons present in an element.The electrons are represented as dots in the structures. The given element is, fluorine which is a group 7A element. The atomic number of fluorine is 17 and fluorine has '7' valence electrons.. The dots represent the number of valence electrons.
How to Draw the Lewis Dot Structure for F2 : Diatomic Fluorine - YouTube A step-by-step explanation of how to draw the F2 Lewis Dot Structure (Diatomic Fluorine).Note that Diatomic Fluorine is often called Molecular Fluorine or ju...
Electron Dot Diagrams | Chemistry for Non-Majors Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element's symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are ...
Lewis Dot Diagram For Fluorine Upon reduction, the fluorine atom forms fluoride, which has 8 valence electrons, and is isoelectronic with a Noble Gas (which one?). A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element.
Fluorine Bohr Model - How to draw Bohr diagram for Fluorine(F) atom Electron dot diagram of Fluorine atom Electron dot diagram also called lewis structure which represents the valence electrons of atoms. As, from the Bohr diagram of Fluorine, we got to know, it has 7 valence electrons. So, just represent these 7 valence electrons around the Fluorine atom as a dot. The electron configuration of Fluorine









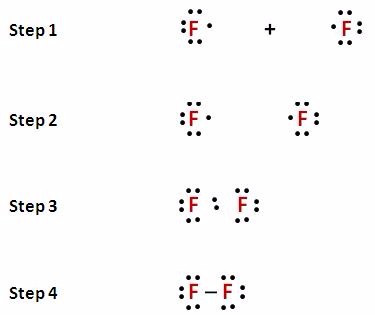
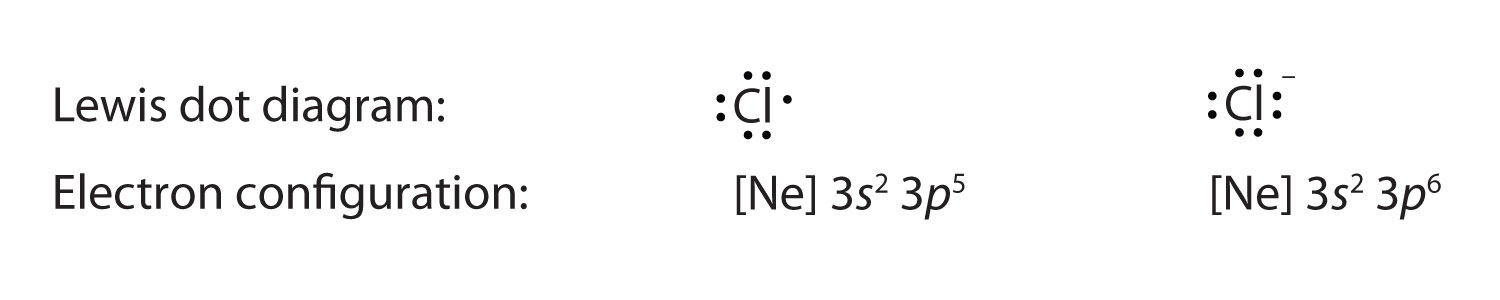






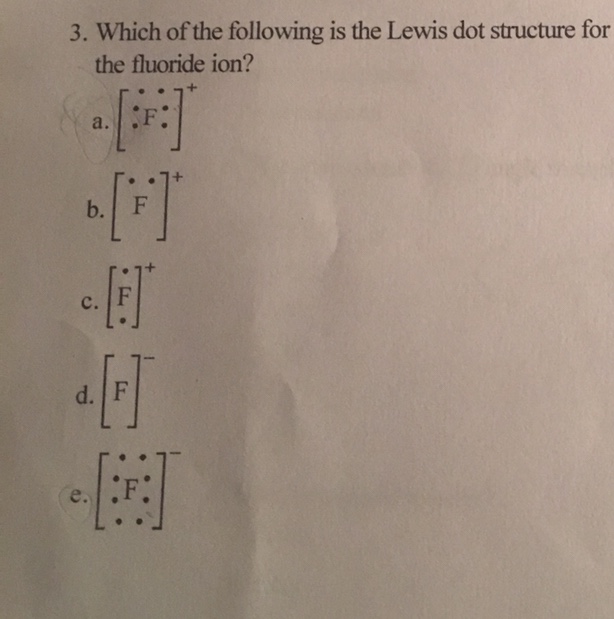


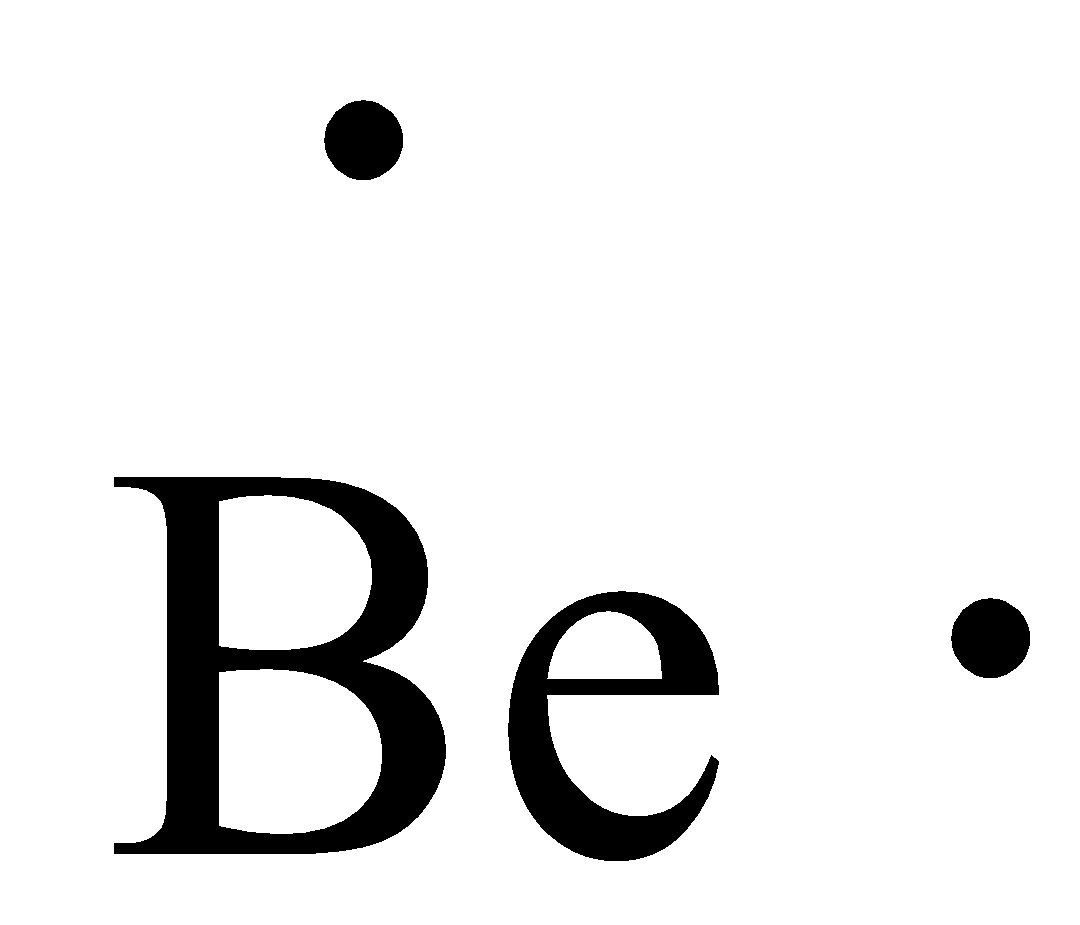




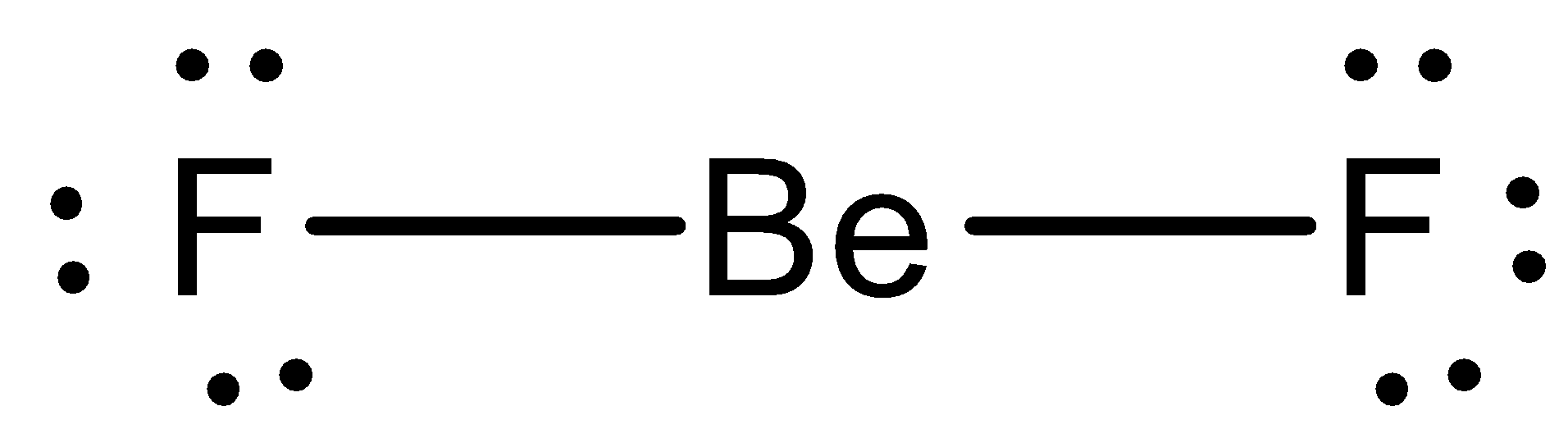

![Expert Answer] draw the electron dot structure of F2 - Brainly.in](https://hi-static.z-dn.net/files/df8/67df4fdbd3d368c6287c82f3548774aa.png)






0 Response to "41 fluorine electron dot diagram"
Post a Comment