42 energy level diagram for oxygen
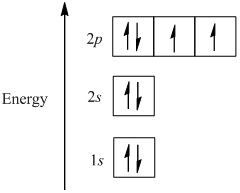
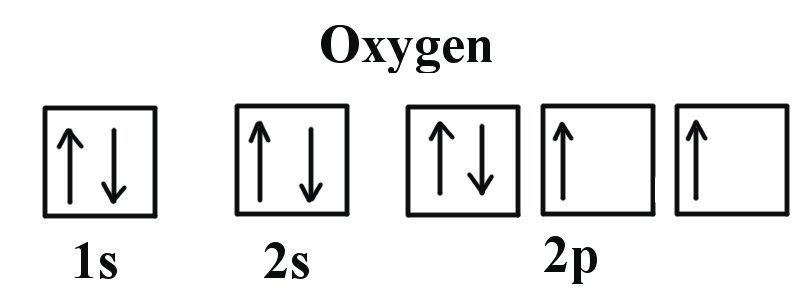
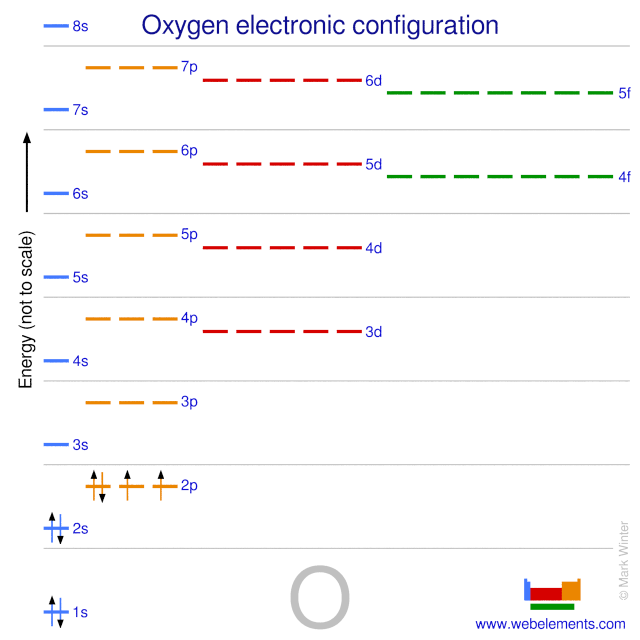
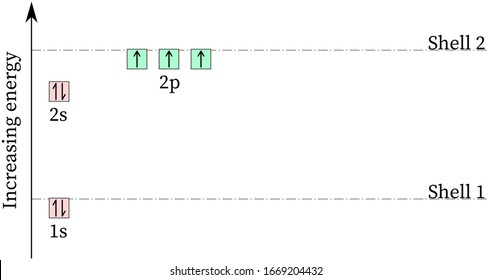
Atomic Data for Oxygen (O ) O I Ground State 1s 2 2s 2 2p 4 3 P 2 Ionization energy 109837.02 cm-1 (13.61805 eV) Ref. MG93 O II Ground State 1s 2 2s 2 2p 3 4 S° 3 / 2 Ionization energy 283270.9 cm-1 (35.1211 eV) Ref. MKM93-1 (35.1211 eV) Ref. MKM93 How to Represent Electrons in an Energy Level Diagram ... You look on the periodic table and find that oxygen is atomic number 8. This number means that oxygen has 8 protons in its nucleus and 8 electrons. So you put 8 electrons into your energy level diagram. You can represent electrons as arrows. If two electrons end up in the same orbital, one arrow faces up and the other faces down.
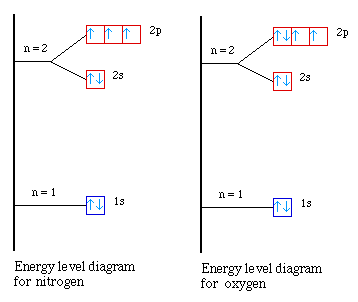
Energy level diagram of nitrogen, oxygen and fluorine ... Energy level diagram of oxygen# nitrogen# fluorine

Energy level diagram for oxygen
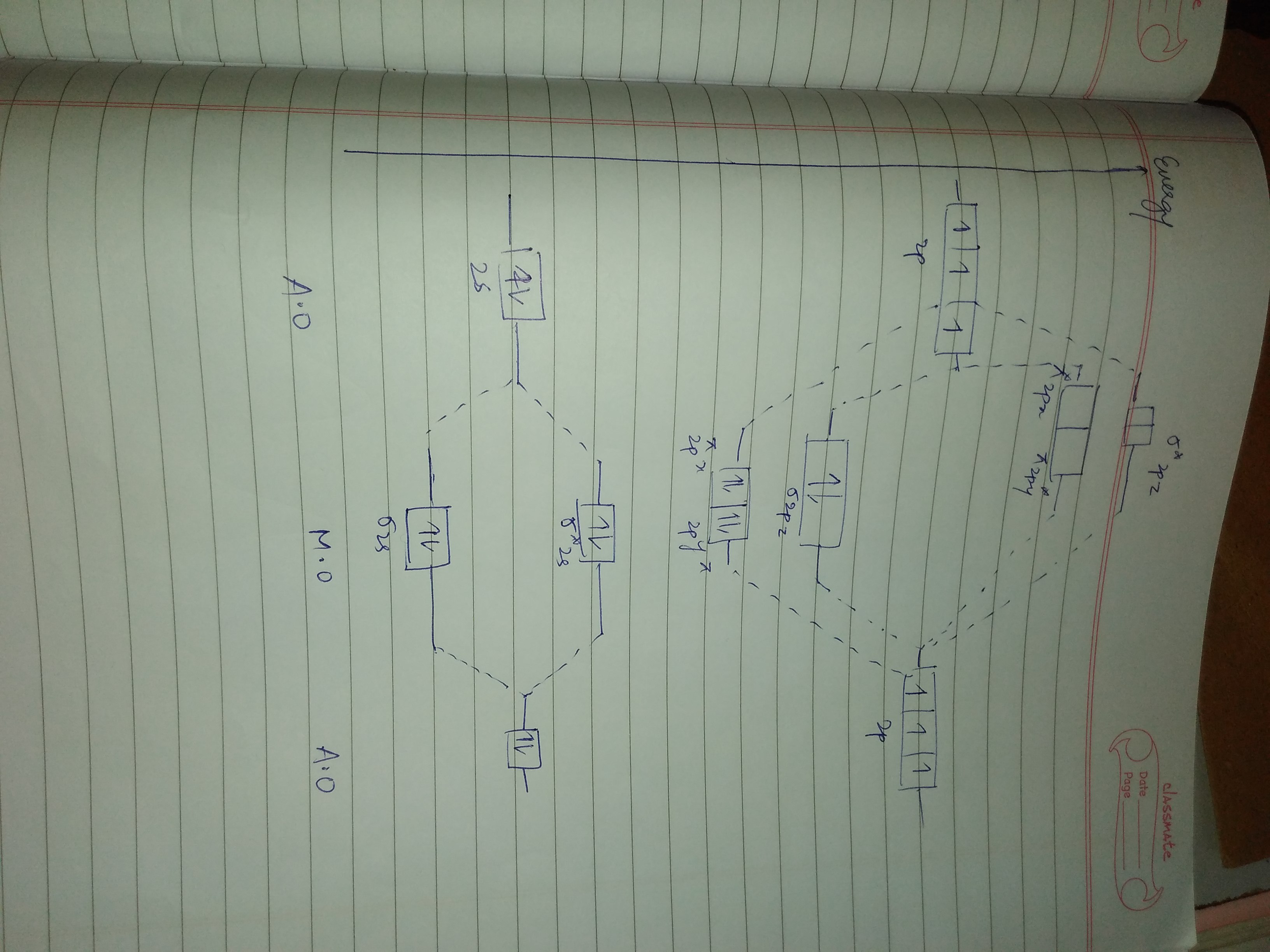
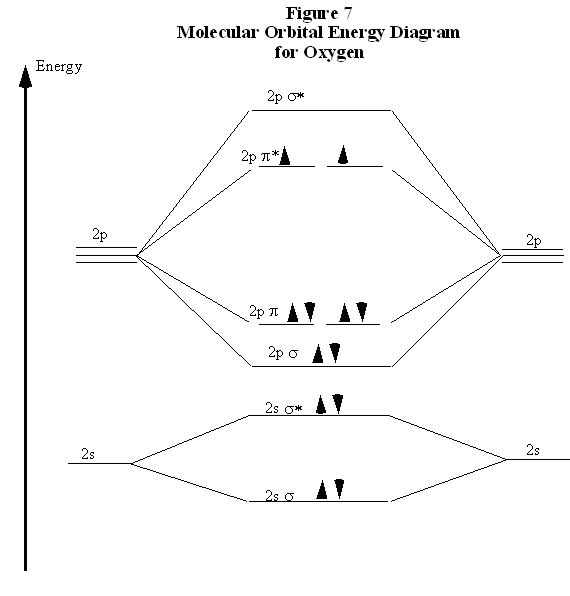
Molecular orbital Energy level diagram of Nitrogen,oxygen ... This video give about what is energy level diagramWe take examples of N,O,CO,NO.. Example Energy level and Orbital box diagrams for Oxygen ... Example energy level and orbital box diagrams for. Example: Energy level and Orbital box diagrams for Oxygen Energy Level Diagram 'Box' Diagram O: 1s 2s 2p Notes: More Examples: Li: C: 218 An electronic configuration is a 'shorthand' version of the atom's respective orbital box diagram . The number of electrons in each orbital or set ... Draw the molecular orbital energy diagram for oxygen ... Zigya App Draw the molecular orbital energy diagram for oxygen molecule (O2) and show that: (i) It has a double bond (ii) It has paramagnetic character. 153 Views Switch Flag Bookmark Discuss in brief sp 2 hybridization (hybridization in C = C bond). Discuss the molecular orbital structure of ethylene (first member of alkene). Or
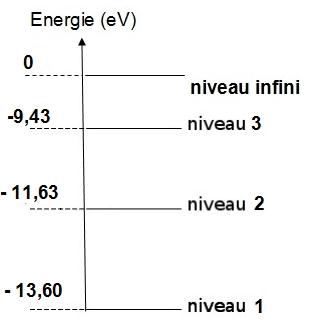
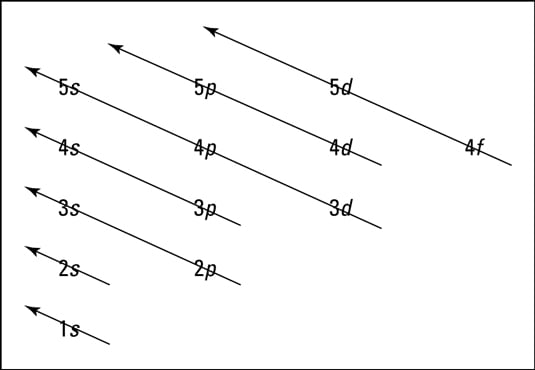
Energy level diagram for oxygen. Energy Level Diagram - Different Energy Shells Around the ... Below is a blank energy level diagram which helps you depict electrons for any specific atom. At energy level 2, there are both s and p orbitals. The 2s has lower energy when compared to 2p. The three dashes in 2p subshells represent the same energy. 4s has lower energy when compared to 3d. Therefore, the order of energy level is as follows: Energy Level Diagram For Hydrogen - Mini Physics The ionization energy of an atom is the energy required to remove the electron completely from the atom. (transition from ground state n = 0 to infinity n = ∞ ). For hydrogen, the ionization energy = 13.6eV. When an excited electron returns to a lower level, it loses an exact amount of energy by emitting a photon. PDF Energy Level Diagrams - the science teacher energy level diagrams Level GCSE (or any course for students aged 14-16) Outcomes 1. To understand the terms exothermic and endothermic 2. To be able to draw energy level diagrams for endothermic and exothermic reactions Information for teachers • These slides take students through a series of steps to help understand energy level diagrams ... Energy Levels, Orbital Diagrams, Electron Config, Noble ... The periodic table shows us energy levels 1 - 7. Energy Levels By default electrons are found in the lowest energy level possible, close to the nucleus. This is called the ground state. When atoms...
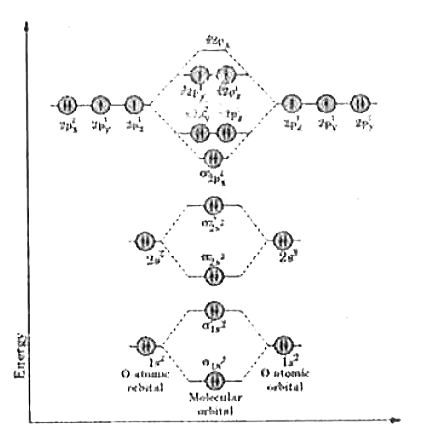
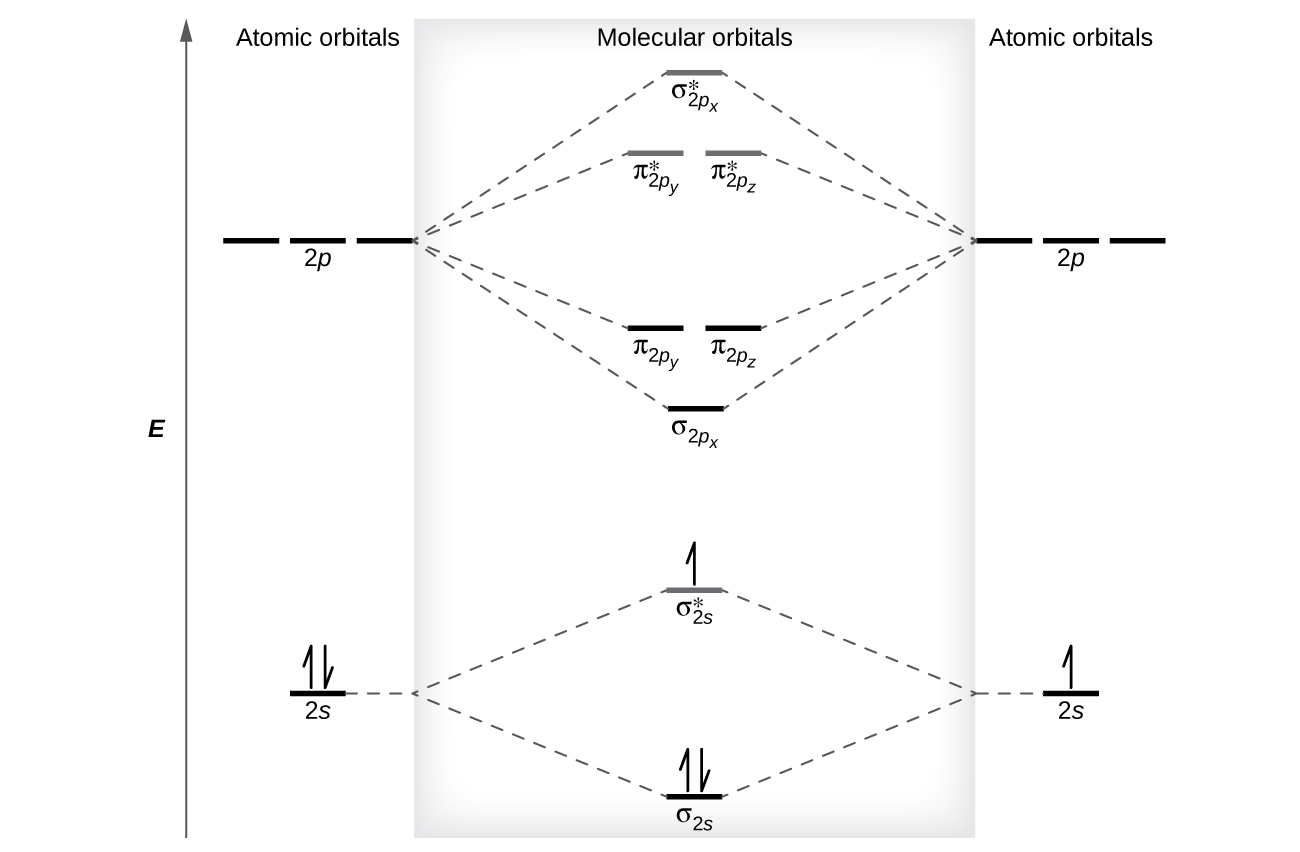
Energy level diagram of Nitrogen and Oxygen - Unit-1 ... Energy level diagram of Nitrogen and Oxygenhttps:// ... Write the Electronic configuration, Energy level diagram ... Jan 22, 2020 · Best answer Atomic number of oxygen = 8. Electronic configuration of oxygen = 1s22s22p4. When two oxygen atoms combines, the molecular orbital energy level diagram is as shown in the figure. From the diagram, the molecular electronic configuration of oxygen is Magnetic property: There are two unpaired electrons Draw the energy level diagram of O2 molecule ... - Sarthaks Draw the M.O diagram for oxygen molecule and calculate its bond order and show that O2 is paramagnetic. asked Dec 17, 2020 in Chemical Bonding by Panna01 ( 47.3k points) chemical bonding Trick to draw energy level diagram for (O2+, O2 ... #MOT #inorganic #chemistry #tricksin this video you can easily to draw energy level diagrams of ionic forms of molecular oxygen.trick to draw energy level di...
Oxygen(O) electron configuration and orbital diagram Scientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there. The electrons of the atom revolve around the nucleus in a certain circular path. These circular paths are called orbit(shell). These orbits are expressed by n. [n = 1,2,3,4 . . . The serial number of the orbit] K is the name of the first orbit, L is the second, M is the third, N is the name of the fourth orbit. The electron holding capacity of each orbit is 2n2. For example, 1. n = 1 for K orbit. The electron holding capacity of K orbit is 2n2 = 2 × 12= 2 electrons. 2. For L orbit, n = 2. The electron holding capacity of the L orbit is 2n2 = 2 × 22= 8 electrons. 3. n=3 for M orbit. The maximum electron holding capacity in M orbit is 2n2 = 2 × 32 = 18 electrons. 4. n=4 for N orbit. The maximum electron holding capacity in N orbit is 2n2 = 2 × 42= 32 electrons. Therefore, the maximum electron holding capacity in the first shell... Sketch of energy-level diagram of molecular oxygen and Si ... Sketch of energy-level diagram of molecular oxygen and Si nanocrystals having the band-gap energies of 1.63 eV and 1.57 eV. Both nanocrystals luminesce at 1.57 eV, while only the first one can... Identifying the Energy Level Diagram for an Oxygen Ion Thus, in total, an oxygen ion contains 10 electrons. The first two of the 10 electrons fill energy level K. The remaining eight electrons fill energy level L. Thus, the electronic configuration is 2,8. And the energy level diagram which represents the correct filling of electrons in the energy levels of an oxygen ion O2− is (B). Hybridization of Atomic Orbitals The two unhybridized p orbitals on carbon form p bonds to the oxygen atoms. The energy diagram for carbon in CO 2 is shown below. What is the hybridization of oxygen in CO 2. Each oxygen has two lone pairs and forms one s bond and one p bond. This means that there must be three hybridized orbitals and one unhybridized p orbital to make the p bond.
Energy Levels of Neutral Oxygen ( O I ) - NIST Oxygen (O) Energy Levels of Neutral Oxygen ( O I ) Configuration : Term : J : Level(cm-1): Ref. 2s 2 2p 4: 3 P: 2: 0.000: MG93 : 1: 158.265
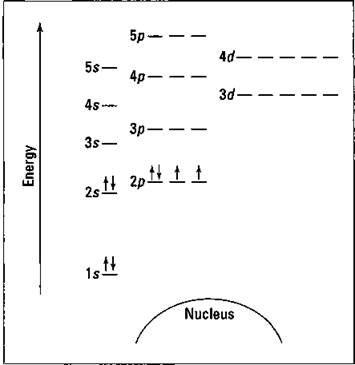
D3.3 Orbital Energy Level Diagrams - Chemistry 109 Fall 2021 D3.3 Orbital Energy Level Diagrams An orbital energy level diagram (or just orbital diagram) shows the relative energies of orbitals and how electrons are distributed among orbitals within a subshell.In an orbital energy level diagram, individual orbitals are usually represented by horizontal lines whose vertical position conveys the qualitative relative energies of the orbitals.
Energy level diagram of oxygen (O2) molecule | Bond order ... This video discuss about the formation of molecular orbitals, their energy levels and filling of electrons in molecule orbitals in Oxygen molecule. Moreover ...
Solved 63. Choose the correct energy level diagram for the ... Choose the correct energy level diagram for the ground state of oxygen. 2p Energy 2s 1s Nucleus 2p 1L 25 Tl Energy 1s Nucleus Energy 2s Nucleus 2 Energy 2s Nucleus 2p Energy 2 1s Nucleus This problem has been solved! See the answer Show transcribed image text Expert Answer 100% (1 rating) answer = b oxygen atomic number 8 electron conf …
Nitrogen energy-level diagram - Big Chemical Encyclopedia Nitrogen energy-level diagram Draw simple molecular orbital energy-level diagrams to indicate how the bonding in the saline hydrides, such as NaH or KH, differs from that between hydrogen and a light p- block element such as carbon or nitrogen. [Pg.741] Figure 6.21 Energy level diagram for nitrogen/oxygen.
Energy level diagram of atomic oxygen showing different ... — Energy level diagram of atomic oxygen showing different spectroscopic transitions related to 1 S and 1 D states. Source publication +7 Coupled Chemistry-Emission Model for Atomic Oxygen Green and...
Energy level diagram of Nitrogen, Oxygen and Fluorine ... Energy level diagram of N2+, N2-| O2+ , 02 -, 02 -2 and F2 | klasspm.comhttps:// ...
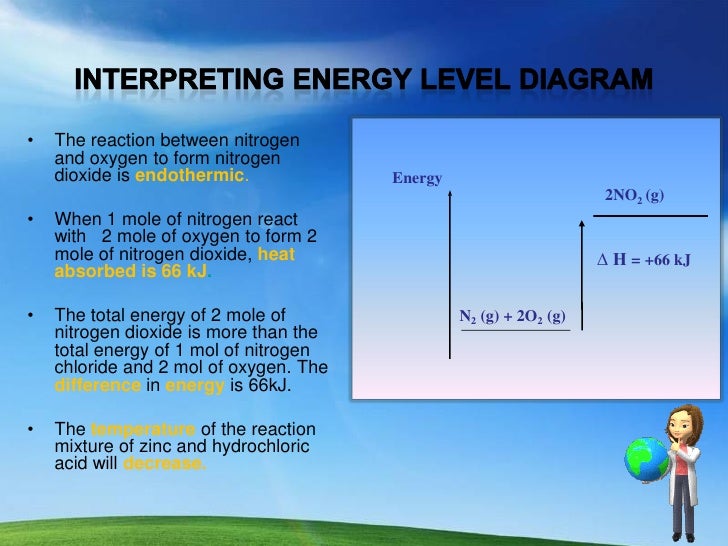
How does the energy level diagram show this reaction is ... Figure shows the energy level diagram for the reaction between methane and oxygen. Based on Figure, the following information can be obtained. (a) The reaction between methane and oxygen to form carbon dioxide and water is an exothermic reaction.
Oxygen Bohr Model - How to draw Bohr diagram for Oxygen(O ... According to the Bohr diagram of Oxygen, the outer shell is L-shell which contains 6 valence electrons. Properties of Oxygen It appears colorless in gas form and pale blue in solid and liquid form. It belongs to period 2, p-block, and group 16 (chalcogens). Its electronegativity is 3.44. It has a cubic crystal structure.
PhysicsLAB: Energy-Level Diagrams Energy level diagrams are a means of analyzing the energies electrons can accept and release as they transition from one accepted orbital to another. These energies differences correspond to the wavelengths of light in the discreet spectral lines emitted by an atom as it goes through de-excitation or by the wavelengths absorbed in an absorption ...
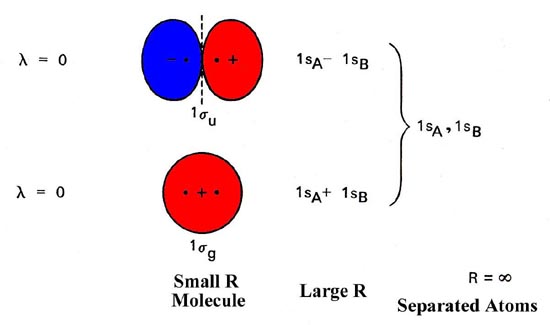
PDF MO Diagrams for Diatomic Molecules MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
Draw the molecular orbital energy diagram for oxygen ... Zigya App Draw the molecular orbital energy diagram for oxygen molecule (O2) and show that: (i) It has a double bond (ii) It has paramagnetic character. 153 Views Switch Flag Bookmark Discuss in brief sp 2 hybridization (hybridization in C = C bond). Discuss the molecular orbital structure of ethylene (first member of alkene). Or
Example Energy level and Orbital box diagrams for Oxygen ... Example energy level and orbital box diagrams for. Example: Energy level and Orbital box diagrams for Oxygen Energy Level Diagram 'Box' Diagram O: 1s 2s 2p Notes: More Examples: Li: C: 218 An electronic configuration is a 'shorthand' version of the atom's respective orbital box diagram . The number of electrons in each orbital or set ...
Molecular orbital Energy level diagram of Nitrogen,oxygen ... This video give about what is energy level diagramWe take examples of N,O,CO,NO..



















0 Response to "42 energy level diagram for oxygen"
Post a Comment