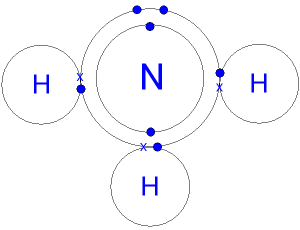
37 the ammonia molecule in the diagram has the observed bond orientation because ...
Covalent bonds hold atoms together because they. ... The ammonia molecule in the diagram has the observed bond orientation b/c? all of the above Rating: 4,5 · 2 reviews
The ammonia molecule in the diagram has the observed bond orientation because ... A. N has four pairs of electrons in the valence shell. Rating: 5 · 3 reviews

The ammonia molecule in the diagram has the observed bond orientation because ...
Two C atoms form a double bond. Eaxh C is bound to two H atoms. Which statement is true? ... The ammonia molecule in the below diagram has the observed orientation because: a. N has 7 protons in its nucleus ... d. the molecule has a bent structure. Show transcribed image text Expert Answer. The ammonia molecule in the diagram has the observed bond orientation because. All of the above a. All of the above a. The ammonia molecule in the diagram has the observed bond orientation because eduhawks april 22 2019 we do your essays write my book report writing a literature review comments off on the ammonia molecule in the diagram has the ... The ammonia molecule in the diagram has the observed bond orientation because ... There is a ball-and-stick model of ammonia, NH3. Three hydrogen atoms are attached to nitrogen. N has 7 protons in its nucleus. N has four pairs of electrons in the valence shell. All of the above. None of the above.
The ammonia molecule in the diagram has the observed bond orientation because .... The ammonia molecule in the diagram has the observed bond orientation because... a) electrons repel one another b) N has four pairs of electrons in the valence shell c) N has 7 protons in its nucleus d) all of the above e) none of the above Nitrogen has a total of 7 protons (its atomic number is 7) in its nucleus. Explanation: The shape and the bond orientation of molecules and ions are both explained by the valences shell electron pair repulsion theory (VSEPR). Ammonia, , is a molecule which contains three N-H bonds, as well as one lone pair on nitrogen. According to the VSEPR ... The binding of urea to the active site of urease has not been observed. Proposed ... 3 molecule. Simultaneously, the bond between the oxygen and the 6-coordinate nickel is broken. This leaves a carbamate ion coordinated to the 5-coordinate Ni, which is then displaced by a water molecule, regenerating the enzyme. The carbamate produced then spontaneously degrades to produce another … Uranus had been observed on many occasions before its recognition as a planet, but it was generally mistaken for a star. Possibly the earliest known observation was by Hipparchos, who in 128 BC might have recorded it as a star for his star catalogue that was later incorporated into Ptolemy's Almagest. The earliest definite sighting was in 1690, when John Flamsteed observed it at least six ...
lower because Mars has lower mass and a smaller radius that together produce a lower gravitational force. Compare the gravity between these pairs, each consisting of an Earth-like planet and its star. You are given the mass of the planet in Earth masses, the mass of the star in Sun masses, and the distance in AUs. lowest to highest gravity: 1 Mearth / 2 Msolar / 2 AU 4 Mearth / 2 Msolar / 3 AU ... In a double covalent bond, a carbon atom shares ... electrons in two orbitals. The ammonia molecule in the diagram has the observed bond orientation because ... The energy from fossil fuels has been recognized as a main factor of global warming and environmental pollution. Therefore, there is an urgent need to replace fossil fuels with clean, cost-effective, long-lasting, and environmentally friendly fuel to solve the future energy crisis of the world. Therefore, the development of clean, sustainable, and renewable energy sources is a prime concern. As we have observed before, nature tends to go to a lower energy state. By analogy, we will consider the driving ... This should be clear since energy has to be added to a molecule of H 2 to break the bond. 1.19 The Formation of New Bonds Releases Energy. Atoms bond together to form compounds because in doing so they attain lower energies than they possess as individual atoms, as indicated by ...
Draw the product of the reaction between ch3chchch3 and h2 under a platinum catalyst. 06.10.2021 · Cholesterol, though it is not an energy molecule, has importance in the body because it _____. helps provide essential nutrients to the brain and lungs helps mobilize fats during periods of starvation is a stabilizing component of the plasma membranes and is the parent molecule of steroid hormones enters the glycolytic pathway without being altered Academia.edu is a platform for academics to share research papers. The ammonia molecule in the diagram has the observed bond orientation because ... electrons repel one another. N has 7 protons in its nucleus.
The ammonia molecule in the diagram has the observed bond orientation because. The ammonia molecule in the diagram has the observed bond orientation because. N has four pairs of electrons in the valence shellb. Electrons repel one another c. Rotation can occur around single bonds. All of the above e. N has 7 protons in its nucleus.

Additive And Emergent Catalytic Properties Of Dimeric Unnatural Amino Acid Derivatives Aldol And Conjugate Additions Gracia Retamosa Chemistry A European Journal Wiley Online Library
Covalent Bonds hold atoms together because they. ... The ammonia molecule in the diagram has the observed bond orientation because.

The Frequency Domain Infrared Spectrum Of Ammonia Encodes Changes In Molecular Dynamics Caused By A Dc Electric Field Pnas
The ammonia molecule in the diagram has the observed bond orientation because ... A. N has four pairs of electrons in the valence shell. B. electrons repel one another. C. N has 7 protons in its nucleus. D. All of the above. E. None of the above.
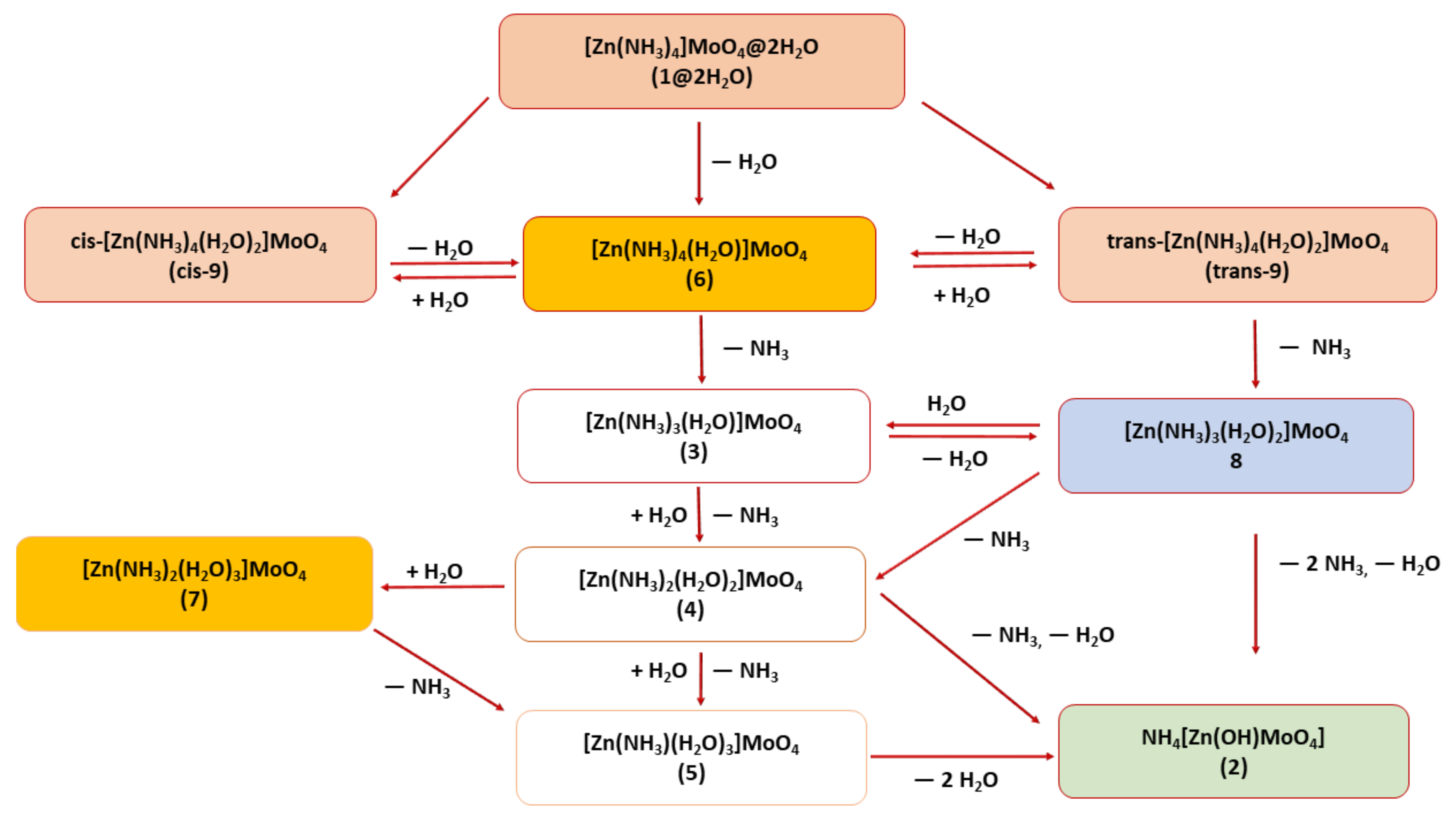
Molecules Free Full Text Solid Phase Self Hydrolysis Of Zn Nh3 4moo4 2h2o Involving Enclathrated Water An Easy Route To A Layered Basic Ammonium Zinc Molybdate Coordination Polymer Html
The ammonia molecule in the diagram has the observed bond orientation because n has four pairs of electrons in the valence shell electrons repel one another and n has 7 protons in its nucleus without making or breaking bonds the pictured molecule can chafe its shape because. A large molecule is broken down or splits to produce salt and water.
The ammonia molecule in the diagram has the observed bond orientation because…All of the above (N has 7 protons in its nucleus, electrons repel one another, ...
The ammonia molecule in the diagram has the observed bond orientation because a. (c) The electrostatic potential diagram of the water molecule. The polarity of the NOH bonds occurs because nitrogen has a greater electronegativity than hydrogen. (b) The dipole moment of the ammonia molecule oriented in an electric field.

Novel Methyl Orange Assisted Core Shell Polyaniline Silver Nanosheets For Highly Sensitive Ammonia Chemiresistors Chaudhary Journal Of Applied Polymer Science Wiley Online Library
of methanal can form hydrogen bonds with water. In the box below, draw a water molecule in a correct orientation to illustrate a hydrogen bond between a molecule of water and the molecule of methanal. Use a dashed line to represent the hydrogen bond. See diagram above. 1 point is earned for a correct diagram.

Calcite Crystal Orientation Patterns In The Bilayers Of Laminated Shells Of Benthic Rotaliid Foraminifera Sciencedirect
The ammonia molecule in the diagram has the observed bond orientation because ... There is a ball-and-stick model of ammonia, NH3. Three hydrogen atoms are attached to nitrogen. N has 7 protons in its nucleus. N has four pairs of electrons in the valence shell. All of the above. None of the above.
The ammonia molecule in the diagram has the observed bond orientation because. All of the above a. All of the above a. The ammonia molecule in the diagram has the observed bond orientation because eduhawks april 22 2019 we do your essays write my book report writing a literature review comments off on the ammonia molecule in the diagram has the ...

The Aerobic And Anaerobic Respiratory Chain Of Escherichia Coli And Salmonella Enterica Enzymes And Energetics Ecosal Plus
Two C atoms form a double bond. Eaxh C is bound to two H atoms. Which statement is true? ... The ammonia molecule in the below diagram has the observed orientation because: a. N has 7 protons in its nucleus ... d. the molecule has a bent structure. Show transcribed image text Expert Answer.
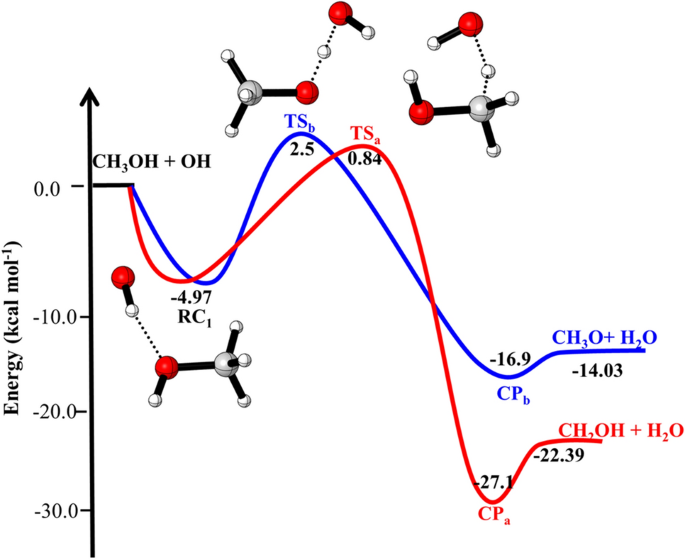
Effect Of Ammonia And Water Molecule On Oh Ch3oh Reaction Under Tropospheric Condition Scientific Reports
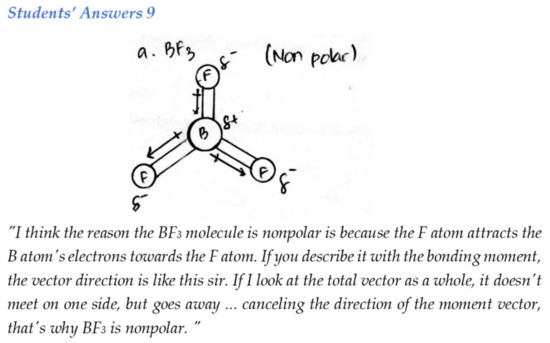
Education Sciences Free Full Text Analysing Students Spatial Abilities In Chemistry Learning Using 3d Virtual Representation Html

The Aerobic And Anaerobic Respiratory Chain Of Escherichia Coli And Salmonella Enterica Enzymes And Energetics Ecosal Plus

A Full Dimensional Ab Initio Potential Energy And Dipole Moment Surfaces For Nh3 2 The Journal Of Chemical Physics Vol 155 No 16

Complexes Of Bis Ortho Cyclometalated Bisphosphinoaryl Ruthenium Ii Cations With Neutral Meta Bisphosphinoarene Ligands Containing An Agostic C H Ru Interaction Organometallics
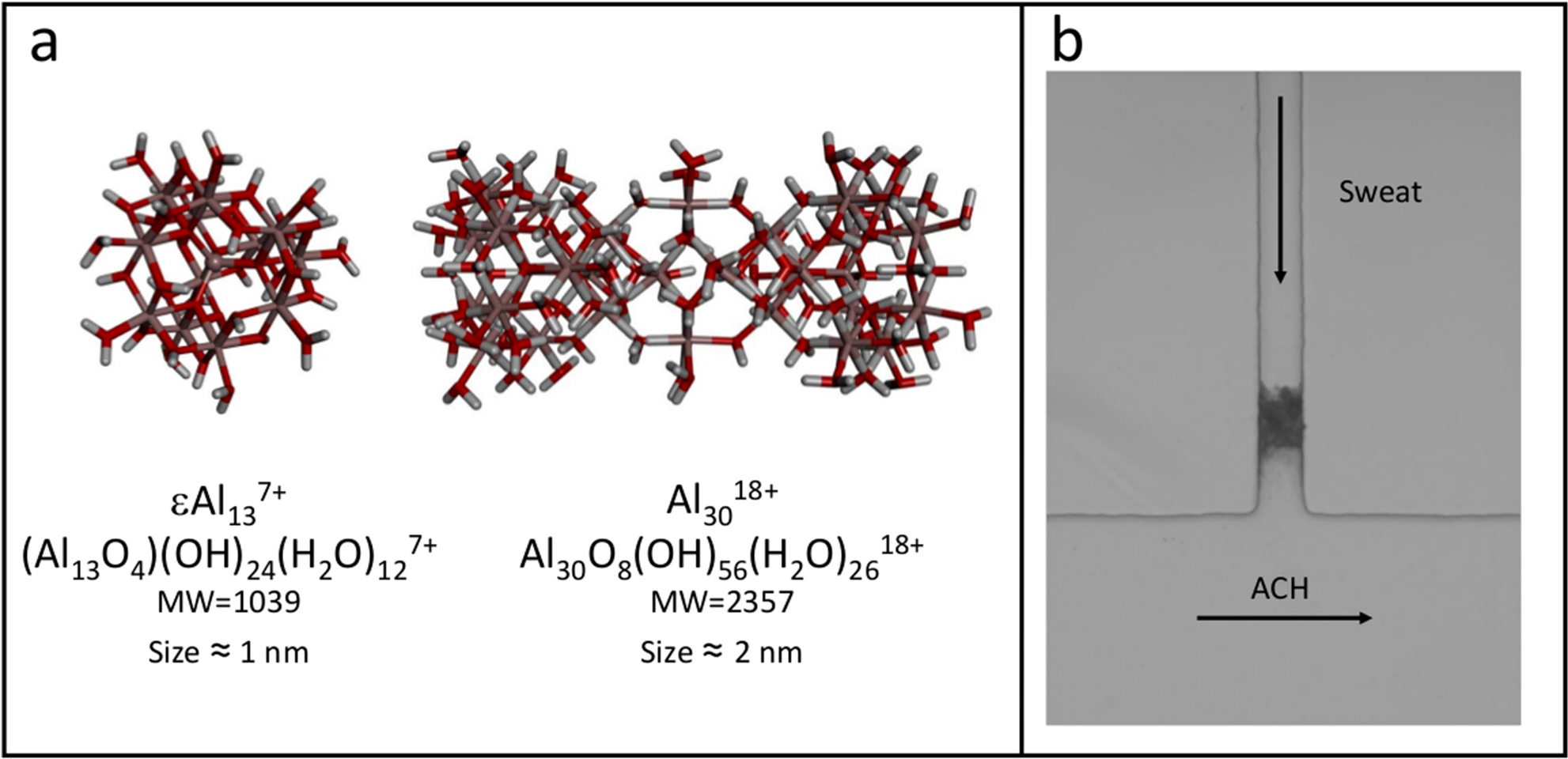
Real Time Observation Of The Interaction Between Aluminium Salts And Sweat Under Microfluidic Conditions Scientific Reports

Material Strategies In The Electrochemical Nitrate Reduction Reaction To Ammonia Production Materials Chemistry Frontiers Rsc Publishing
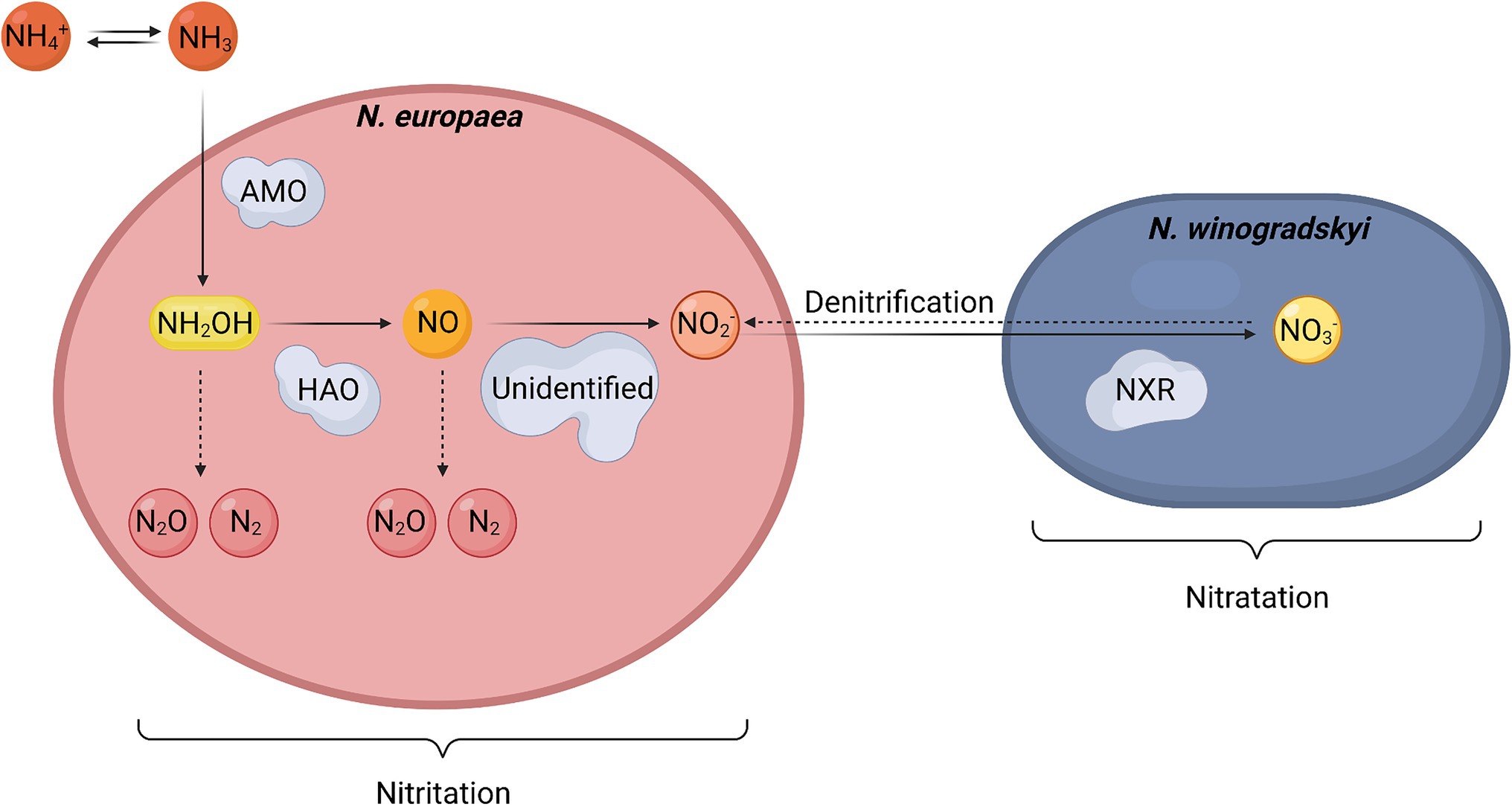
Frontiers Development Of Nitrogen Recycling Strategies For Bioregenerative Life Support Systems In Space Microbiology
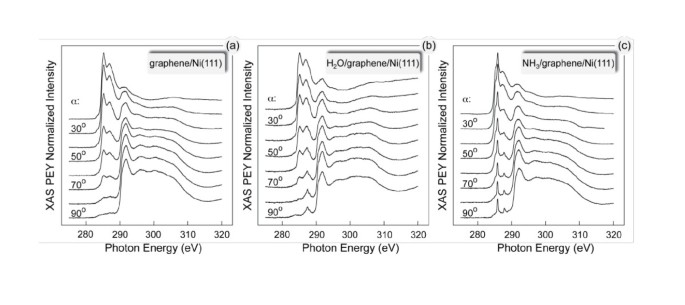
Graphene On Ferromagnetic Surfaces And Its Functionalization With Water And Ammonia Nanoscale Research Letters Full Text
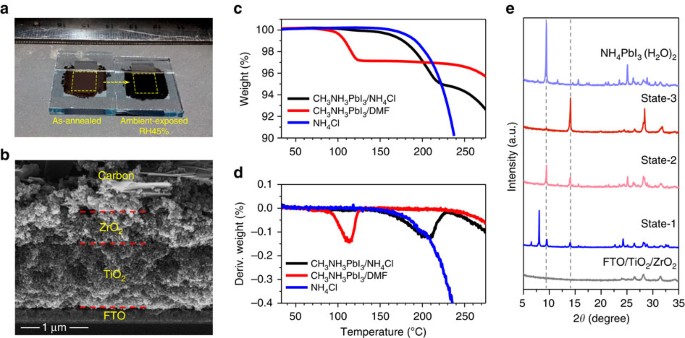
Synergy Of Ammonium Chloride And Moisture On Perovskite Crystallization For Efficient Printable Mesoscopic Solar Cells Nature Communications




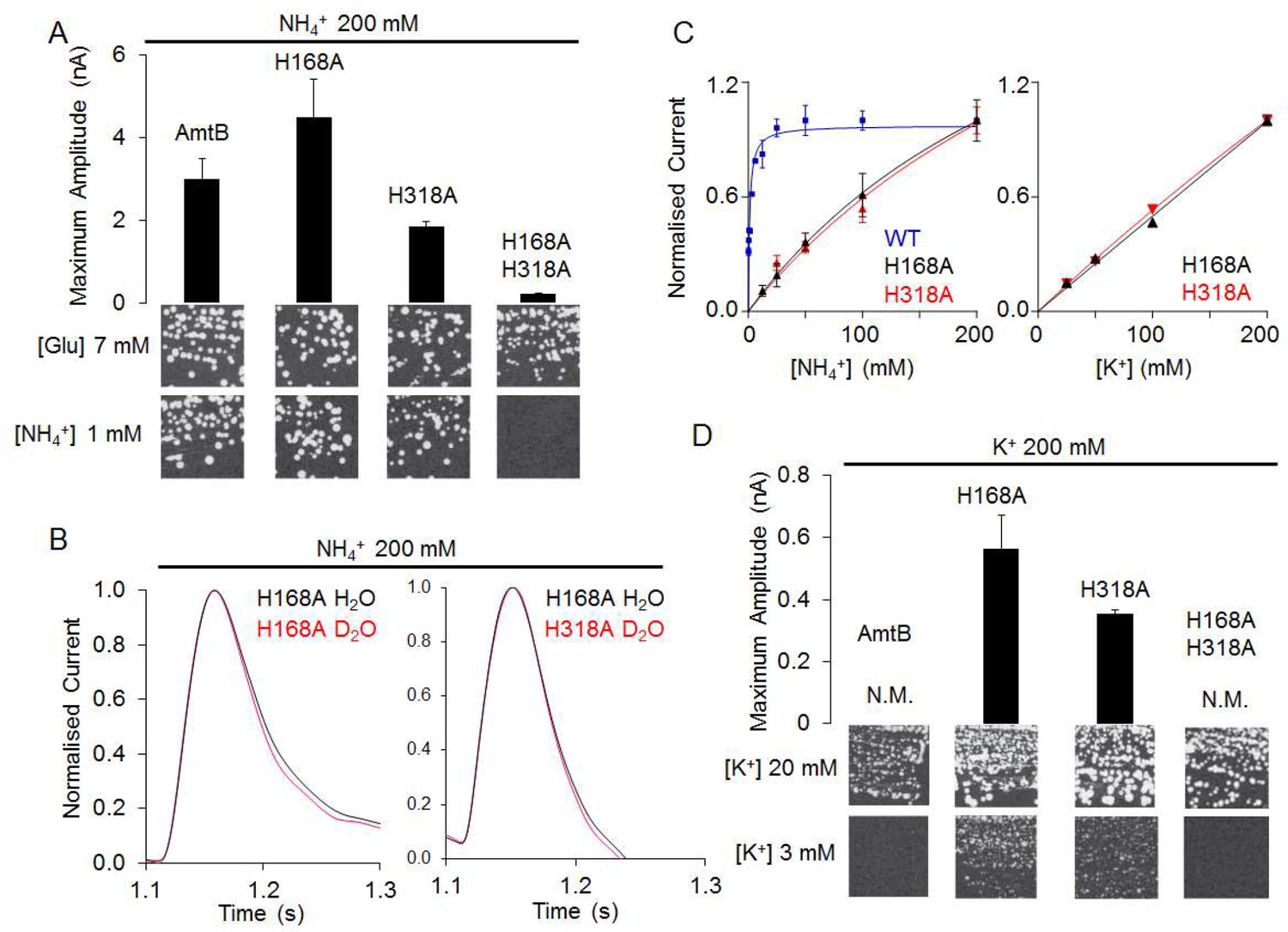










0 Response to "37 the ammonia molecule in the diagram has the observed bond orientation because ..."
Post a Comment