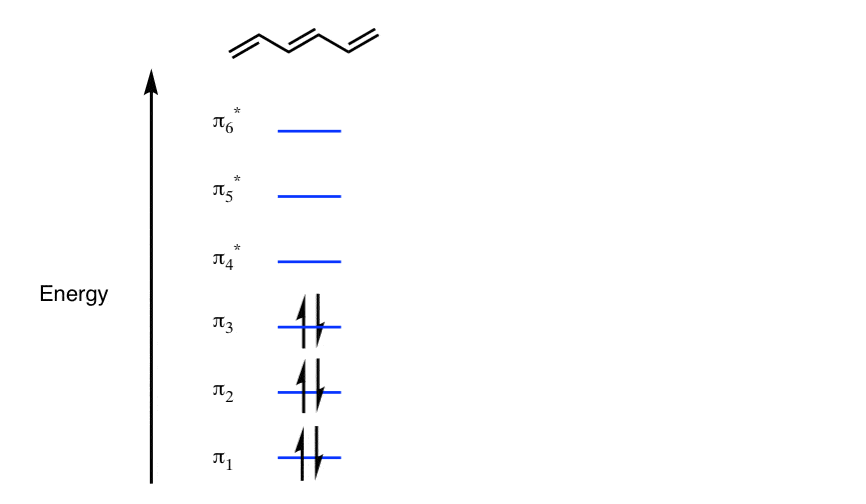
39 partial energy level diagram for hydrogen
The energy levels agree with the earlier Bohr model, and agree with experiment within a small fraction of an electron volt. If you look at the hydrogen energy levels at extremely high resolution, you do find evidence of some other small effects on the energy. The 2p level is split into a pair of lines by the spin-orbit effect. Powered by FlexBook® textbook Platform ® © CK-12 Foundation 2021; Please wait... Please wait...
Download scientific diagram | A simplified energy-level diagram of negative hydrogen and negative deuterium ͑ not to scale ͒ . The origin of the energy axis ...
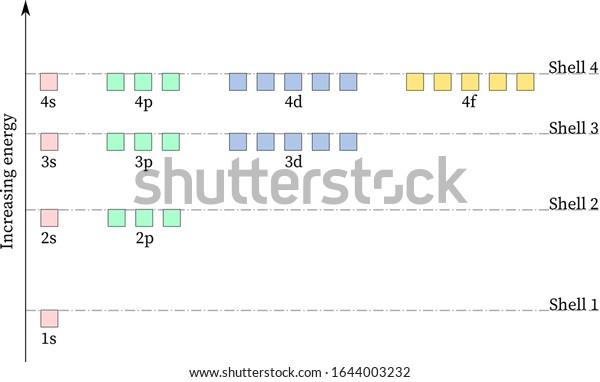
Partial energy level diagram for hydrogen
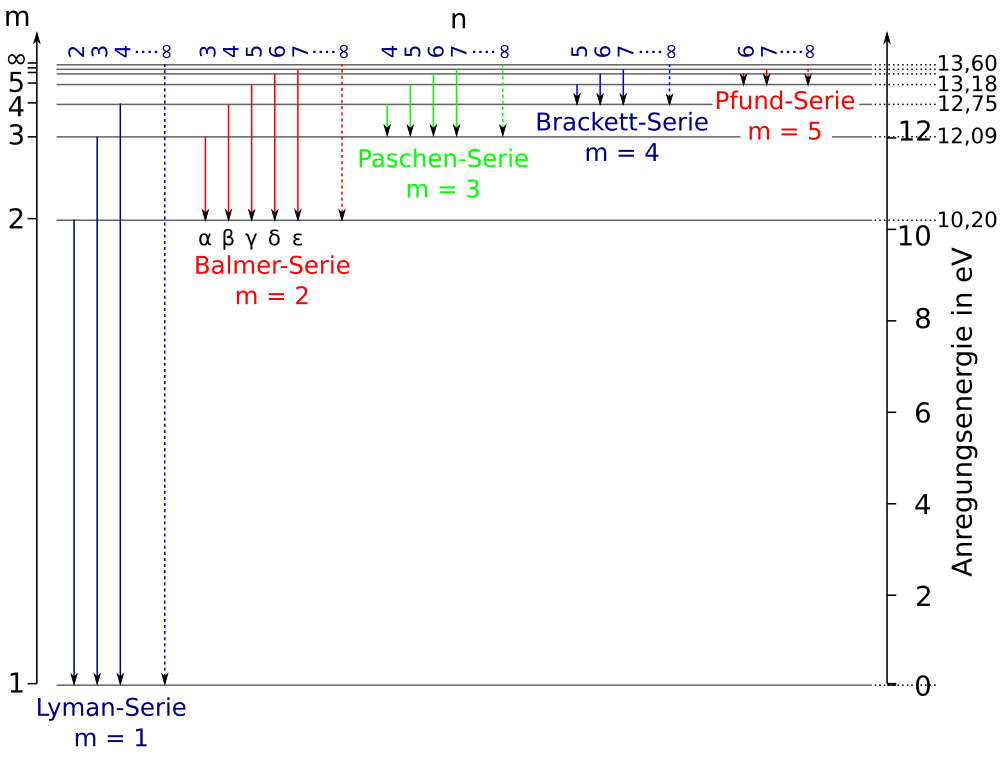
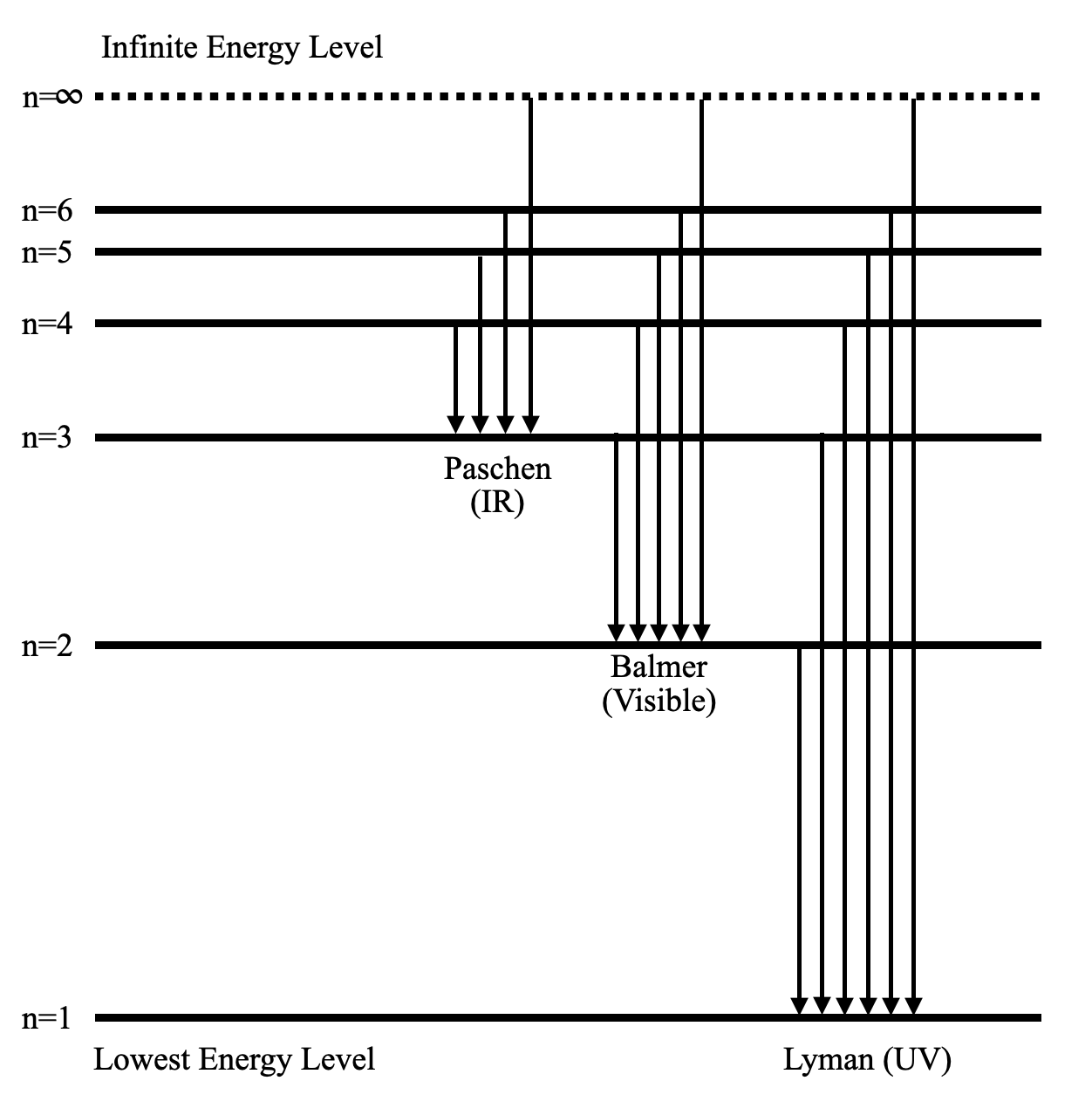
The ionization energy of an atom is the energy required to remove the electron completely from the atom. (transition from ground state n = 0 to infinity n = ∞ ). For hydrogen, the ionization energy = 13.6eV. When an excited electron returns to a lower level, it loses an exact amount of energy by emitting a photon. View Lab Report - Partial Energy Level Diagram for Hydrogen from CHEM 2070 at Cornell University. Partial Energy Level Diagram for Hydrogen 1 = (6.626 x 10-37 KJ) (3.00 x 1017 nm/s) (6.022 x 1023 Energy level diagrams and the hydrogen atom. It's often helpful to draw a diagram showing the energy levels for the particular element you're interested in. The diagram for hydrogen is shown above. The n = 1 state is known as the ground state, while higher n states are known as excited states. If the electron in the atom makes a transition from ...
Partial energy level diagram for hydrogen. a) Draw a diagram of energy levels to explain the spectrum of lines of the hydrogen atom. b) Indicate, for each photon, that its region can be emitted to the electromagnetic spectrum. c) Compare, in a graph, the energies of the orbital of the hydrogen atom with the energies of the He +. The ground state energy level of the electron in a hydrogen atom is −13.6 eV, which is equivalent to an ultraviolet photon of roughly 91 nm wavelength.. The energy levels of hydrogen can be calculated fairly accurately using the Bohr model of the atom, which conceptualizes the electron as "orbiting" the proton in analogy to the Earth's orbit of the Sun. 1 answerThe hydrogen atom is the simplest in the universe that consists of only a proton and an electron. The electron is at the ground state, which is the nearest ... For hydrogen-like atoms (ions) only, the Rydberg levels depend only on the principal quantum number n. ... The energy level of the bonding orbitals is lower, and the energy level of the antibonding orbitals is higher. For the bond in the molecule to be stable, the covalent bonding electrons occupy the lower energy bonding orbital, which may be signified by such symbols as σ or π depending on ...
01.06.2018 · Hydrogen vehicle tanks nowadays operate at 5000–10,000 psi . Compressed hydrogen is a highly efficient methodology for hydrogen storage and the energy density considering volumetric increase with the pressure increase of the gas. However, the targeted efficiency of the gas depends on a low gravimetrically and volumetrically. Unlike the energy levels of the harmonic oscillator potential, which are evenly spaced by ħω, the Morse potential level spacing decreases as the energy approaches the dissociation energy. The dissociation energy D e is larger than the true energy required for dissociation D 0 due to the zero point energy of the lowest (v = 0) vibrational level. Partial oxidation cannot be used for gasifying gasoline, diesel, methanol, or ethanol, because of the decrease in energy content of the fuel. However, the hydrogen-rich gas (hence, the preference for this type of process in the petroleum industry) that is produced by partial oxidation may be used to enrich other fuels. For the production of ... Partial Energy Level Diagram for Hydrogen: Energy (J/atom) -1 x 10-19 -2x 10-19 -3 x 10-19 -4 x 10-19 -5 x 10-19 -6 x 10-19 Previous question Next question COMPANY
Partial energy level diagram for hydrogen. 41126 43477 48710 65842. The ionization energy of an atom is the energy required to remove the electron completely from the atomtransition from ground state n 0 to infinity n. Hydrogen partial energy level diagram selfhomeworkhelp submitted 7 years ago by evomax01 i did a spectroscopy lab and i need to ... Does anyone know where I can find Partial Energy Level Diagram for Hydrogen? Thanxs. Answers and Replies Mar 24, 2005 #2 dextercioby. Science Advisor. Homework Helper. Insights Author. 13,149 710. Click here to get an answer to your question ✍️ Draw a neat labelled energy level diagram of the Hydrogen atom.19 Nov 20191 answer · Top answer: Given figure shows energy level diagram for Hydrogen atom. I did a spectroscopy lab and I need to construct a partial energy-level diagram for hydrogen.
energy-level diagram for the NO molecule. We assume that orbital order is the same as that for N2. The bond order is 2.5. Figure 9.42: The molecular orbital energy-level diagram for both the NO+ and CN-ions. Figure 9.43: A partial molecular orbital energy-level diagram for the HF molecule.
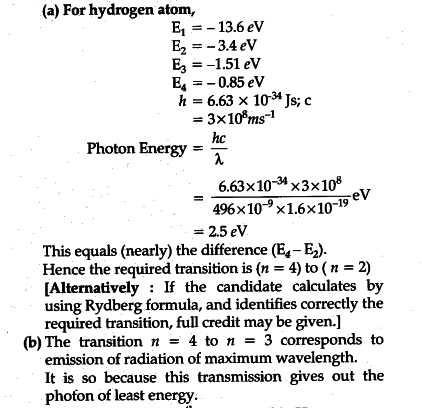
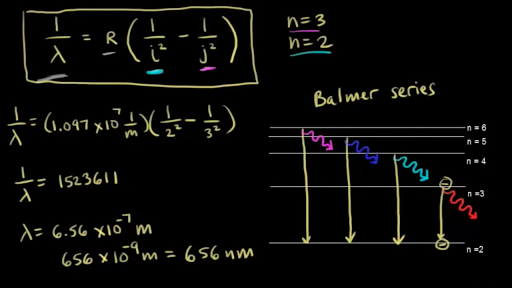
Click here👆to get an answer to your question ️ The following question relates to the partial energy level diagram for the hydrogen electron: .54eV n5 .85eV n4 1.51eV n3 3.4eV n2 13.6eV n1 The question relates to a hydrogen electron located at E3 .What is the emission energy when the electron falls to E5 from E3 ?
The energy level of an atom is the amount of energy contained within corresponding orbitals. Explore the definition of energy levels, study the Bohr Model of an atom, and learn how to use an ...
The overall process of ethanol fermentation followed by partial oxidation reforming has a practical hydrogen production yield similar to that of MFCs, but lower overall energy efficiency than MFCs because of more energy losses during microbial fermentation, distillation, and chemical reforming (Zhang, 2011a). Overall, these microbe-based biological transformations suffer from low hydrogen ...
For the partial energy-level diagram of hydrogen, assume that all the observed transitions terminate at the n = 2 state; for example, if you observe two ...
Energy storage is the capture of energy produced at one time for use at a later time to reduce imbalances between energy demand and energy production. A device that stores energy is generally called an accumulator or battery.Energy comes in multiple forms including radiation, chemical, gravitational potential, electrical potential, electricity, elevated temperature, latent heat and kinetic.
Energy level diagrams and the hydrogen atom. It's often helpful to draw a diagram showing the energy levels for the particular element you're interested in. The diagram for hydrogen is shown above. The n = 1 state is known as the ground state, while higher n states are known as excited states. If the electron in the atom makes a transition from ...
View Lab Report - Partial Energy Level Diagram for Hydrogen from CHEM 2070 at Cornell University. Partial Energy Level Diagram for Hydrogen 1 = (6.626 x 10-37 KJ) (3.00 x 1017 nm/s) (6.022 x 1023
The ionization energy of an atom is the energy required to remove the electron completely from the atom. (transition from ground state n = 0 to infinity n = ∞ ). For hydrogen, the ionization energy = 13.6eV. When an excited electron returns to a lower level, it loses an exact amount of energy by emitting a photon.

The Figure Shows An Energy Level Diagram For The Hydrogen Atom Several Transitions Are Marked As I Ii Iii The Diagram Is Only Indicative And Not Be Scale Img Src Https D10lpgp6xz60nq Cloudfront Net Physics Images

Draw A Neat And Labelled Energy Level Diagram And Explain Balmer Series And Brackett Series Of Spectral Lines For Hydrogen Atom The Work Function For A Metal Surface Is 2 2 Ev If Light


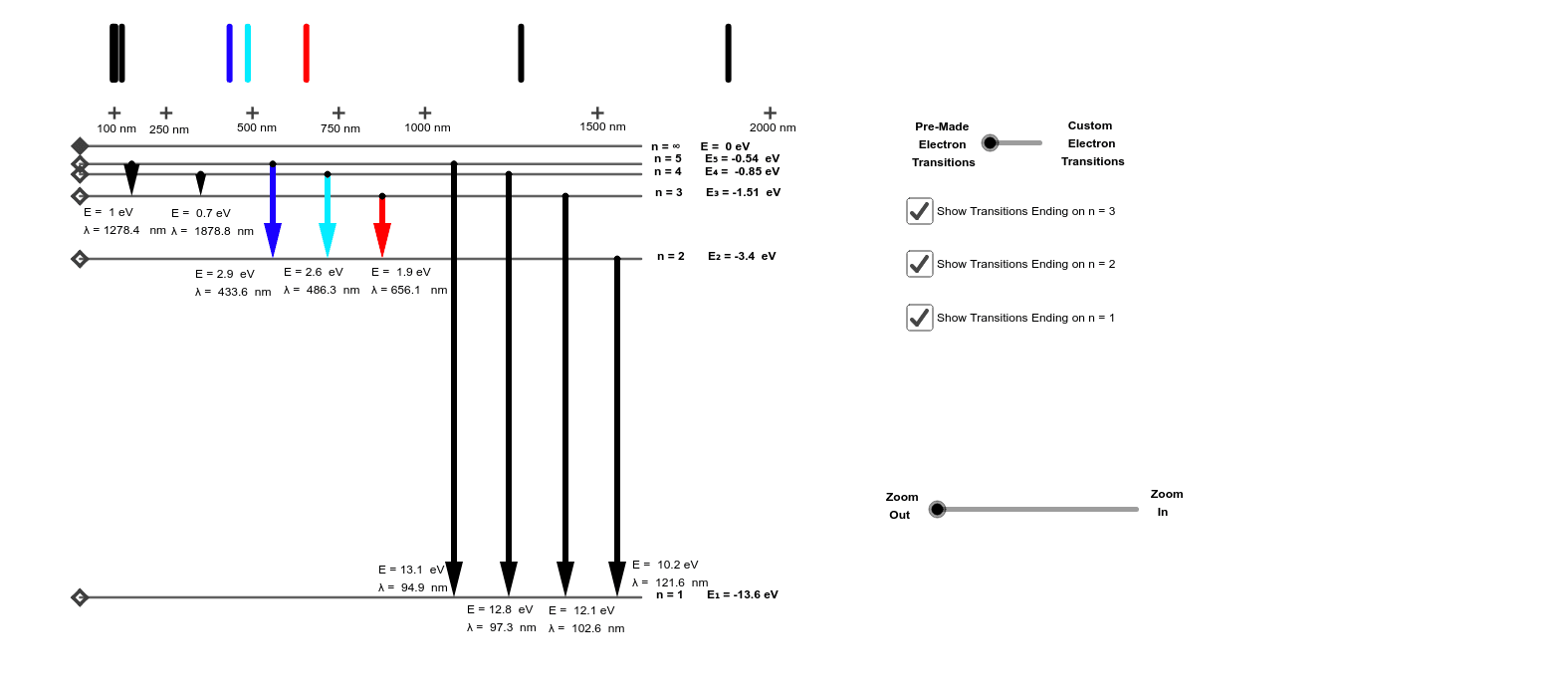



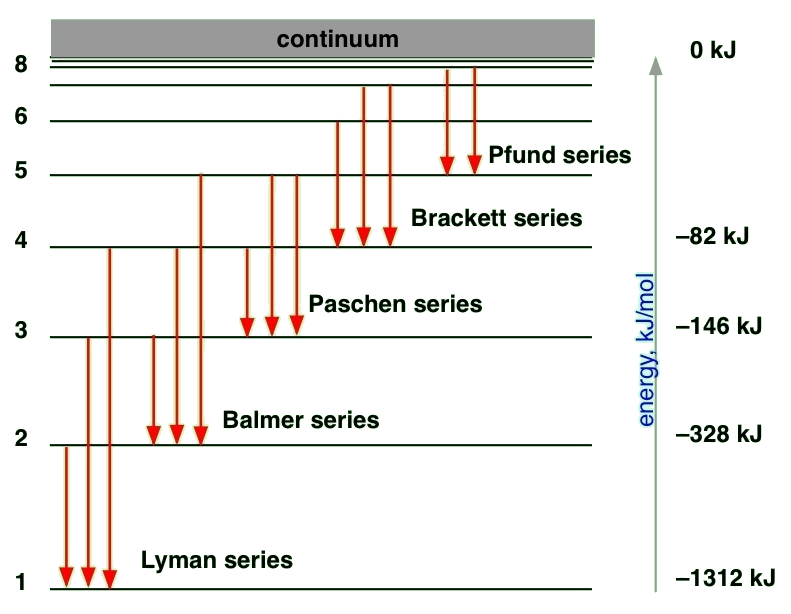



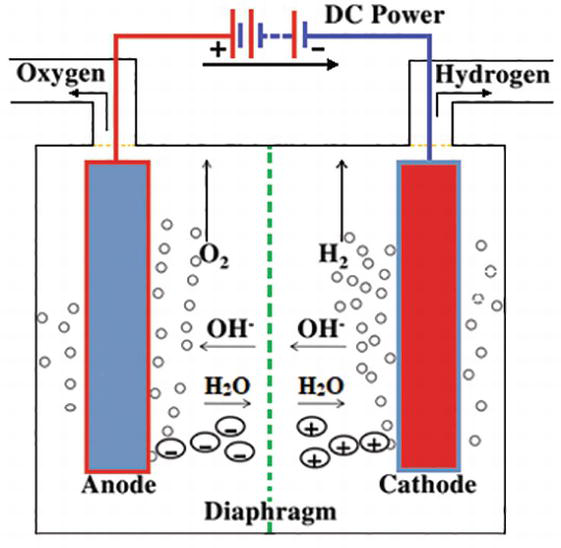





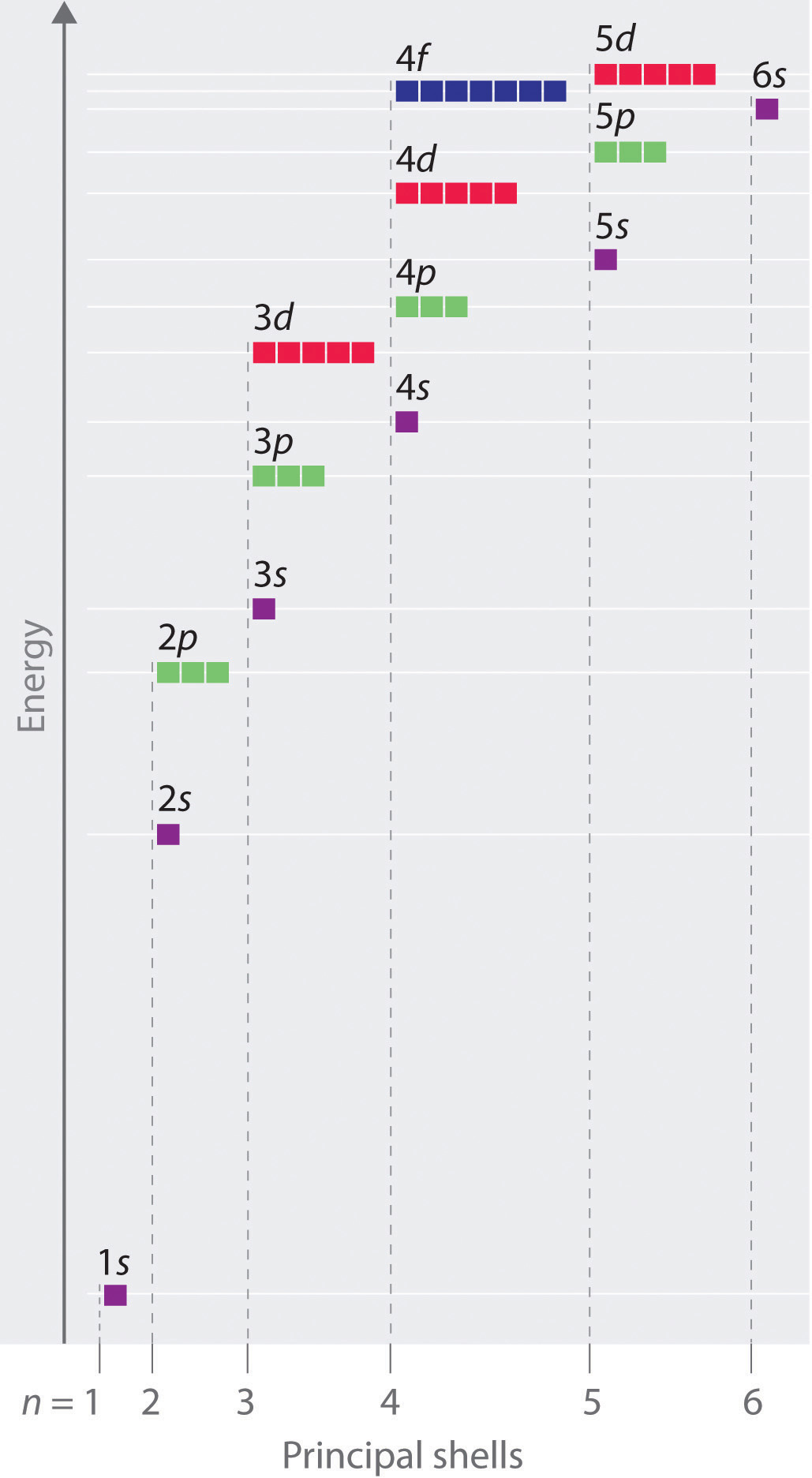

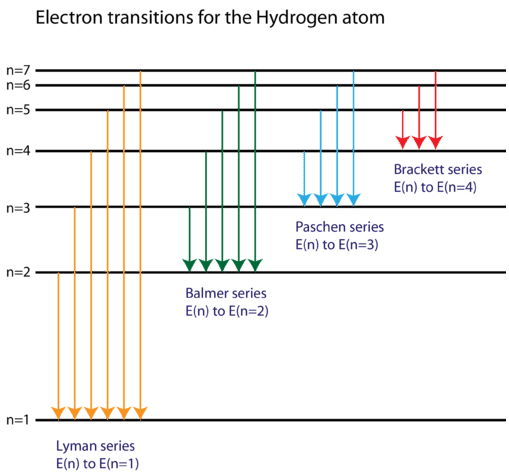
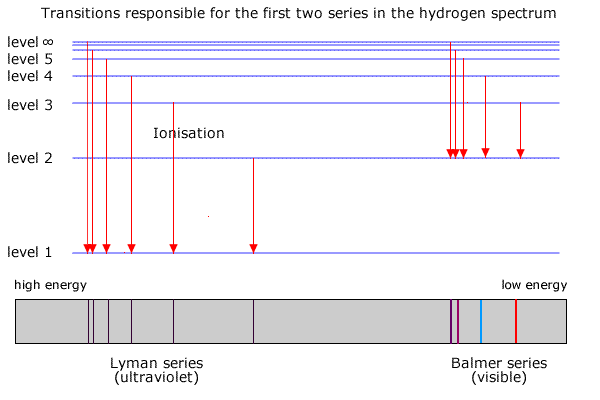


0 Response to "39 partial energy level diagram for hydrogen"
Post a Comment