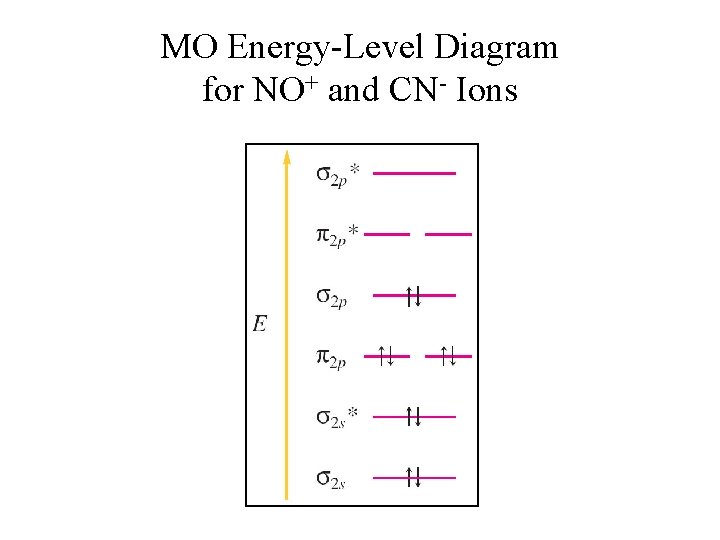
40 no+ molecular orbital diagram
Here's the question >Paramagnetic materials have unpaired electrons and are weakly attracted by magnetic fields, whereas diamagnetic materials have no unpaired electrons and are weakly repelled by such fields. By considering the molecular orbital diagram of the “diatomic” molecules below, determine which are paramagnetic. The options are then diatomic boron, carbon, nitrogen, oxygen, and fluorine. The problem is that I have no idea how to tell if something has unpaired electrons. My und... ***Sweeper knew many things.*** It's true! Over his long existence, he had learned so much. He had seen stars forming and dying, had felt the magnetic field of a magnetar and the gravity of a singularity, had travelled further than anyone he'd ever met, had even seen hints of a greater truth, hidden far beyond the material and even the immaterial. But still, all he learned only made it ever clearer how little he actually knew. Sweeper was not built for scientific inquiry, his mind did not natu...
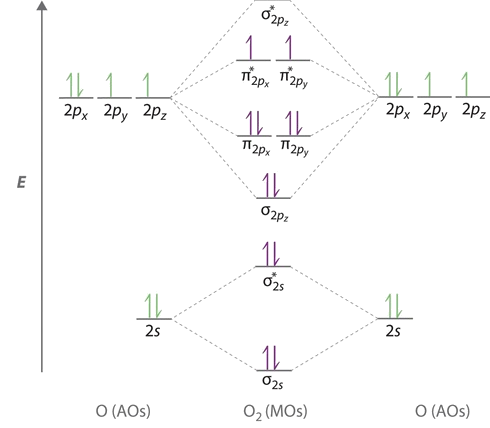
Bond order can be calculated by the difference between the bonding electrons and non-bonding electrons in the molecular orbitals. For example, O=O has a bond ...1 answer · Top answer: Bond order of NO++ The atomic number of N is 7 and the atomic number of O is 8. There are five valence electrons present in nitrogen and...

No+ molecular orbital diagram
The MO Diagram predicts a paramagentic molecule. Heteronuclear Diatomic MO Diagrams. Question 1. MO Diagram of NO+. NO+ Structures. Both the Lewis Structure and ... orbitals. Answer: The molecular orbital diagram for CO and NO+ molecule and ion are: The bond order is the difference between the number of the bonding ...1 page \[[https://pubchem.ncbi.nlm.nih.gov/periodic-table/png/Periodic\_Table\_of\_Elements\_w\_Chemical\_Group\_Block\_PubChem.png](https://pubchem.ncbi.nlm.nih.gov/periodic-table/png/Periodic_Table_of_Elements_w_Chemical_Group_Block_PubChem.png) \] or \[[https://ptable.com/#Properties](https://ptable.com/#Properties) \] In the last post, I mentioned the concept of activation barriers: reactions require an energy input to proceed from starting materials to a transition state even if there is a net re...
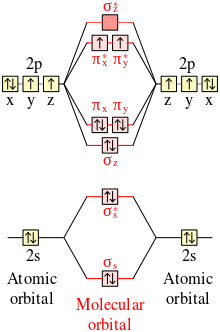
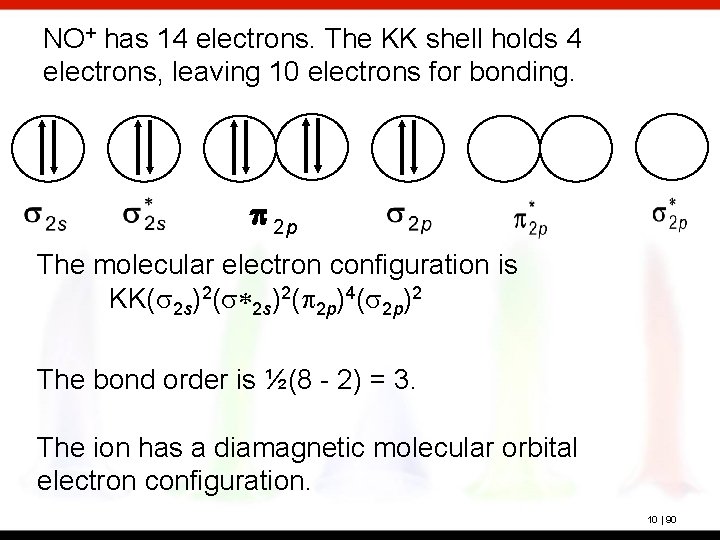
No+ molecular orbital diagram. I need some help. I understand these molecules based on molecular orbital diagrams, but my homework is asking to determine magnetism on VBT CN- NO NO+ OF- Bond order for NO+ is 1/2(8-2)=3. NO has 11 valence e- (8 bonding, 3 antibonding): Bond order for NO is 1/2(8-3)=2.5. And NO- has 12 bonding e- (8 bonding, 4 antibonding): Bond order for NO- is 1/2(8-4)=2. So you can see there is an increasing bond order in the form of: NO-<NO<NO+. In addition to this the bond lengths will be: NO+=106pm, NO=115pm & NO-=127pm Jul 02, 2020 · 1. 199. Molecular Orbital Diagram of NO. TAGS. Molecular Orbital Diagram. Previous article Molecular Orbital Diagram of CO. Next article Qualitative and Quantitative Analysis |Organic Chemistry. Lecture On Molecular Orbital Diagram Of NO,NO+ And HCl Molecules Which Is Explained In Details..Molecular Orbital Energy Level Diagramhttps://youtu.be/Go6VyX...
NO+. Bond order = 3 shortest bond (106 pm). NO Bond order = 2.5 intermediate (115 pm) ... molecular orbitals in the diagram suggest a double bond.29 pages ¤ Molecular Orbital diagram of BN, CN, CN-https://youtu.be/DlIQsEEvicc¤Molecular Orbital diagram of COhttps://youtu.be/8mufOTgvagU¤ S-P mixing OF ORBITALS ... - In order to find the bond order, we need to have information about the molecular orbital diagram of the molecule. Molecular orbital diagram shows which ... NO- has 12 valence electrons: Same as NO but change ^1 at the end to ^2. Your MO diagram for NO should look like this:.3 answers · Top answer: NO+ has 10 valence electrons: (Sigma2s)^2(Sigma*2s)^2(Pi2px,Pi2py)^4(Sigma2pz)^2 NO has 11 ...
A guide for alien Species concerning Humans by a Human &#x200B; Written in Union Standard Machine Communication Language (USMCL) by Henry Katou Vizex Machine Translator recommended for reading &#x200B; Section 1: Common misconceptions regarding Hummans &#x200B; I have met individuals of a grand total of 3 non-Human Species and have already discovered a plethora of astonishing misconceptions about my Species. I am a humble molecular network engineer, but I would like to offer t... We have solutions for your book! Draw the molecular orbital diagrams for NO - and NO +. Compare the bond orders in these two ions. Molecular orbitals are formed by linear combination of atomic orbitals. Atomic orbitals and molecular orbitals of a molecule can be shown in a molecular orbital diagram. Nov 15, 2016 · Molecular Orbital Diagram of NO+. In the link above chem_mod said it was best to account for the negative charge of CN- by placing an extra electron on the nitrogen since it is more electronegative. I was just wondering if the same applied for molecules with a positive charge. Hi /r/Chempros. Have you ever shed blood and tears on writing a script, only to find after a few weeks that something really similar had already been done? Have you ever created a specific tool but didn't really had the time or the right place to share it with your colleagues? Have you ever seen a really useful reddit post that you wish you had saved? I have, and after a quick exchange with our dear mod /u/wildfyr I've decided to post this thread. #Scope I would like for it to be a location ...
My question is asking to describe the bonding in the NO2- ion using valnece bond theory versus Molecular Orbital theory. I know that the ion is bent and that there are 2 sigma bonds and 1 pi bond and that the nitrogen is sp2 hybridized. But the latter part of my question asks how MO theory describes the pi bond in the species. This is a general chem course and I frankly have no clue how to go about drawing a MO diagram for a non-diatomic molecule, so I’m guessing there’s something else I have...
Adrienne H. Chemistry 101. 3 months, 2 weeks ago. Consider the molecules NO+ and and N2 + . Use molecular orbital theory to answer the ...13 Aug 20214 answers · Top answer: its first drop, the molecular orbital diagrams for and Plus and two plus Kendall Plus has five ...
I’m working through a problem set for one of my classes and so far it’s had me make the SALCs and molecular orbital diagram for NH3. Now it’s asking me to go from constructing the MO diagram to identifying the ground electronic state of the molecule. I’m guessing what I need is some kind of wave function to describe the population of each orbital in the ground state. But I’m not sure how to get there from where I am. Any tips are appreciated. I also have to work out which transitions from the ...
Cross posted from chem help so forgive me if for some reason you’re seeing this twice now. I’m working through a problem set for one of my classes and so far it’s had me make the SALCs and molecular orbital diagram for NH3. Now it’s asking me to go from constructing the MO diagram to identifying the ground electronic state of the molecule. I’m guessing what I need is some kind of wave function to describe the population of each orbital in the ground state. But I’m not sure how to get there fro...
15 Nov 2016 — Molecular Orbital Diagram of NO+ ... In the link above chem_mod said it was best to account for the negative charge of CN- by placing an extra ...
[Book 1 of The HEL Jumper](https://www.reddit.com/7oulr8) [Book 2 of The HEL Jumper](https://www.reddit.com/akws1r) \----- [Previous](https://redd.it/idztze) | [First](https://www.reddit.com/eo9svn) | [Next](https://redd.it/iqrcc1) | [Patreon](https://www.patreon.com/SabatonBabylon) Thanks to Big_Papa_Dakky, Darth_Android, bloblob, AMERICUH, The_Real_Jumper, Mr_Polygon, Krystalin, Damned_Thrice, Mamish, Vikairious, Sam_Berry, RedHawkdude, KillTech, LilLaussa, Daddy_Talon, Gruecifer, Gaelan_D...
\[[https://pubchem.ncbi.nlm.nih.gov/periodic-table/png/Periodic\_Table\_of\_Elements\_w\_Chemical\_Group\_Block\_PubChem.png](https://pubchem.ncbi.nlm.nih.gov/periodic-table/png/Periodic_Table_of_Elements_w_Chemical_Group_Block_PubChem.png) \] or \[[https://ptable.com/#Properties](https://ptable.com/#Properties) \] In the last post, I mentioned the concept of activation barriers: reactions require an energy input to proceed from starting materials to a transition state even if there is a net re...
orbitals. Answer: The molecular orbital diagram for CO and NO+ molecule and ion are: The bond order is the difference between the number of the bonding ...1 page
The MO Diagram predicts a paramagentic molecule. Heteronuclear Diatomic MO Diagrams. Question 1. MO Diagram of NO+. NO+ Structures. Both the Lewis Structure and ...


















0 Response to "40 no+ molecular orbital diagram"
Post a Comment