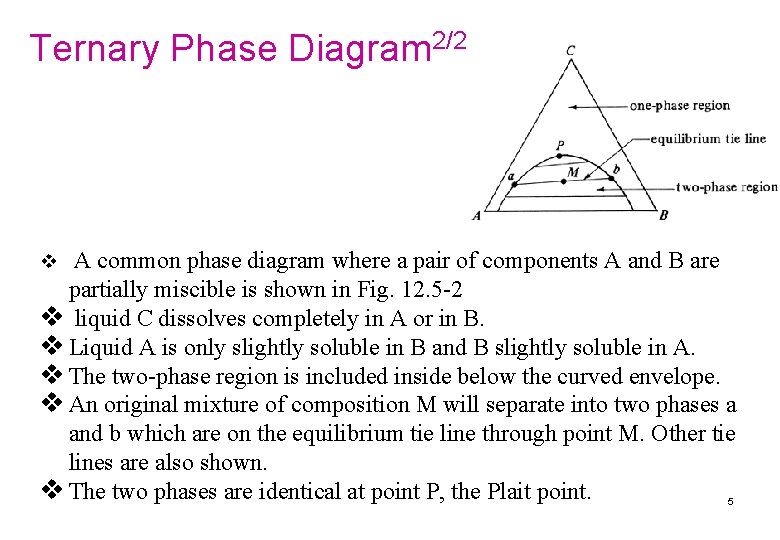
42 lever rule ternary phase diagram
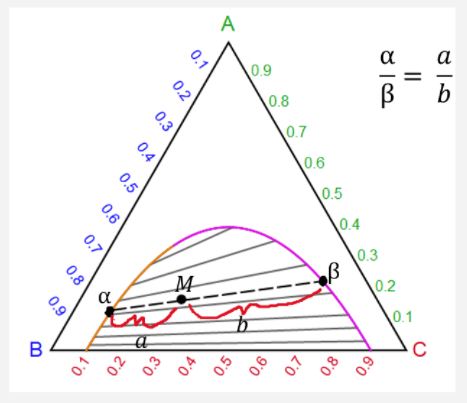
Lecture Notes - The Lever Rule • The definition of the scale of a composition axis is generally chosen so that the values (e.g. ... triangular or ternary composition diagram. The attached ternary diagram is contoured (sequentially) in terms of the three plotting coordinates [B/(A+B+C)], [C/(A+B+C)], and 3- The percentage of crystals formed at any point can be determined using the lever rule. For example, on the ternary diagram sphene - anorthite - wollastonite (Fig. 5), at a temperature represented by point "B", the % of crystals formed from a melt of composition "A" will be: % crystals = 100[AB/BC] (Fig. 5).
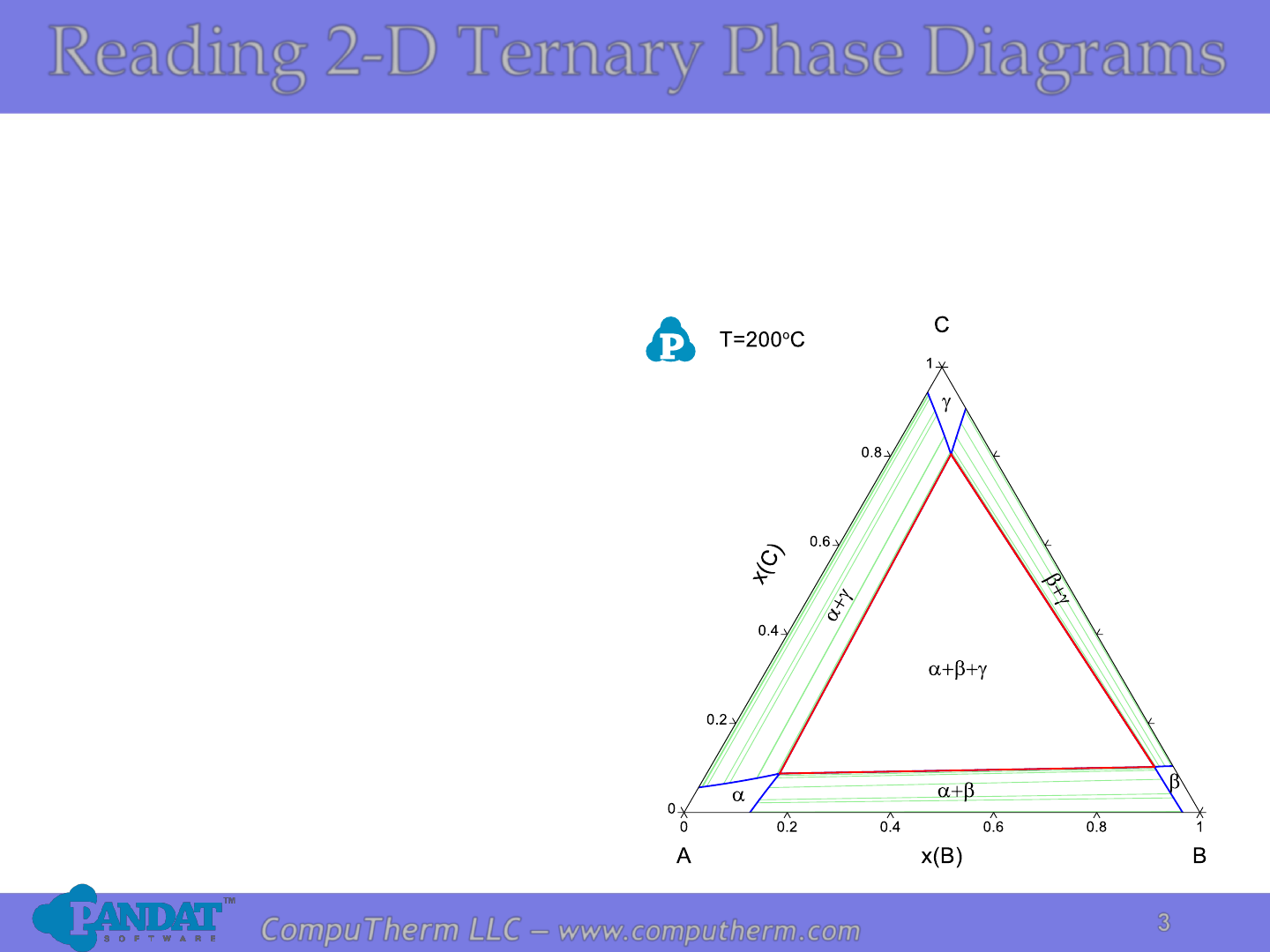
The lever rule in three phase region is graphically illustrated by the weighted phase fractions distributed about the average composition. Figure 31-5: Example of a simple ternary phase diagram at constant and .
Lever rule ternary phase diagram
The lever rule is a rule used to determine the mole fraction (x i) or the mass fraction (w i) of each phase of a binary equilibrium phase diagram. It can be used to determine the fraction of liquid and solid phases for a given binary composition and temperature that is between the liquidus and solidus line. Lever arm rule ternary diagram. Figure 1 gives a 3d view of the ternary phase diagram of this system. Like binary phase diagram you could use lever rule to find the amounts of liquid and solid in a two phase field if the composition of the alloy is known. Ternary phase diagrams lesley cornish. Ternary systems are those having three components. 50 Fresh s How to Read Ternary Phase Diagram. ternary plot a ternary plot ternary graph the first method is an estimation based upon the phase diagram grid the concentration of each species is. Applying the Phase Rule to Ternary Diagrams. ternary diagram basics ternary diagram basics engineer clearly loading mod 01 lec 25 ternary phase diagram ...
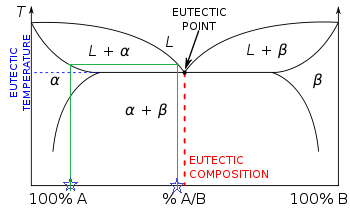
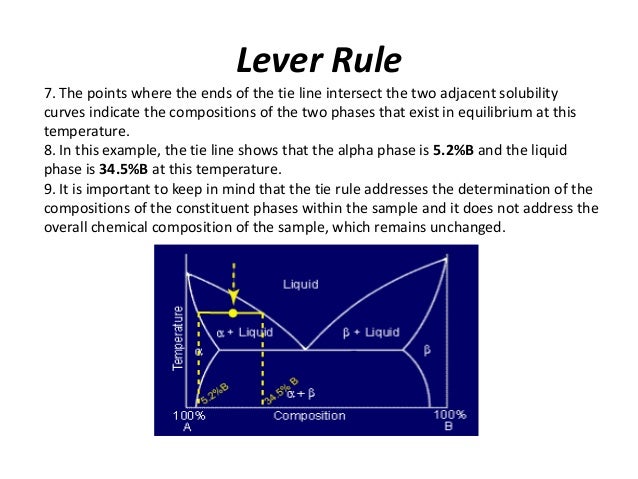
Lever rule ternary phase diagram. Binary phase diagrams and Gibbs free energy curves • Binary solutions with unlimited solubility • Relative proportion of phases (tie lines and the lever principle) • Development of microstructure in isomorphous alloys • Binary eutectic systems (limited solid solubility) • Solid state reactions (eutectoid, peritectoid reactions) • Binary systems with intermediate phases/compounds The lever rule. If an alloy consists of more than one phase, the amount of each phase present can be found by applying the lever rule to the phase diagram. The lever rule can be explained by considering a simple balance. The composition of the alloy is represented by the fulcrum, and the compositions of the two phases by the ends of a bar. Ternary phase diagrams are important for liquid-liquid extraction and are used in the Hunter-Nash method to determine the number of stages required for separations. This module is intended for material and energy balances, thermodynamics, and separations courses. Be able to apply the lever rule to determine the amounts of two phases in equilibrium. It is convenient to use Fig. 3.5 B to illustrate the application of the lever rule in ternary systems. As before, it may be of interest to know the amount of any phase present at a particular temperature, x, for a fixed bulk composition, c. Construction lines xA and xC can be drawn to construct the triangle xAC.
Ternary phase diagram books by D.R.F. West - there are several . Ternary grid . ... ≡ Lever Rule . Not so useful, although . mathematically correct. Not all the compositions . Might lie in this section! Why vertical sections . are limited: Binary eutectic in a ternary system: Start studying Phase Rule. Learn vocabulary, terms, and more with flashcards, games, and other study tools. Search. ... Effect of Pressure on Phase Diagrams where pressure is no a variable... Ternary Diagrams...; Subjects. Arts and Humanities. Languages. Math. Science. Social Science. Other. Features. A point in the two-phase region of a phase diagram indicates not only qualitatively that the liquid and vapor are present, but represents quantitatively the relative amounts of each. To find the relative amounts of two phases a & b in equilibrium, we measure distances on the tie line, l a and l b between the two phases and use the lever rule: Applies the lever rule to a solid-liquid mixture to determine the fraction of each phase in equilibrium and explains the basis for the lever rule. Made by fa...
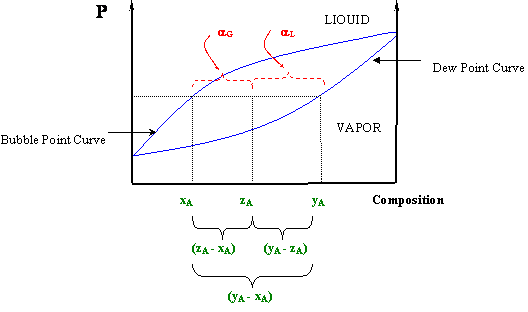
Ternary Phase Diagram Mod-01 Lec-15 Errors in drawing phase diagramsPhase Behaviour of Polymer Blends and Copolymers Muddiest Point- Phase Diagrams I: Eutectic Calculations and Lever Rule Phase Diagrams Nptel Ternary Phase Diagram Software Free Download Ternary April 20th, 2019 - Ternary Phase Diagram Software SoluCalc v 0 1 6 SoluCalc is designed as an Open Source application that has been written by Java and can be used for phase diagram calculation of electrolyte solutions Ternary Phase Diagram an overview ScienceDirect Topics Lever Rule Applied to Phase Diagram for Partially Miscible Liquids Lisa M. Goss; Ternary Phase Diagram with Alternate Phase Envelope Megan E. Maguire and Rachael L. Baumann; Tie Lines from a Conjugate Curve in Ternary LLE Diagram Housam Binous, Farrukh Shehzad, Abdelmalek Hasseine, and Ahmed Bellagi; Apply the Hunter-Nash Method to Liquid ... Sometimes it is also known as the "reverse arm rule," because for the calculation of α L (liquid) you use the "arm" within the (y A -x A) segment closest to the vapor, and for the vapor calculation (α G) you use the "arm" closest to the liquid. Figure 5.5: The Lever Rule In a P-x Diagram. At this point you will see clearly why we ...
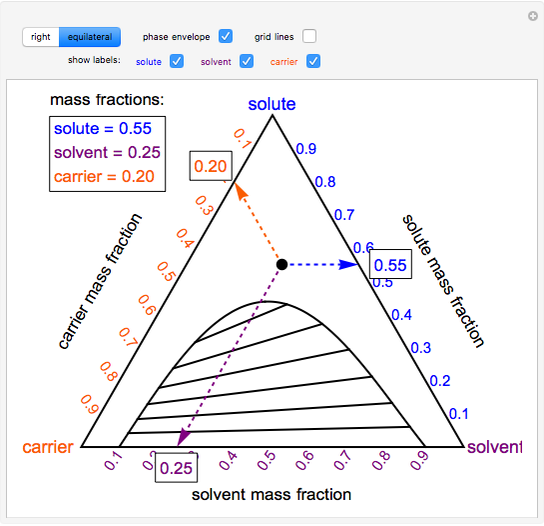
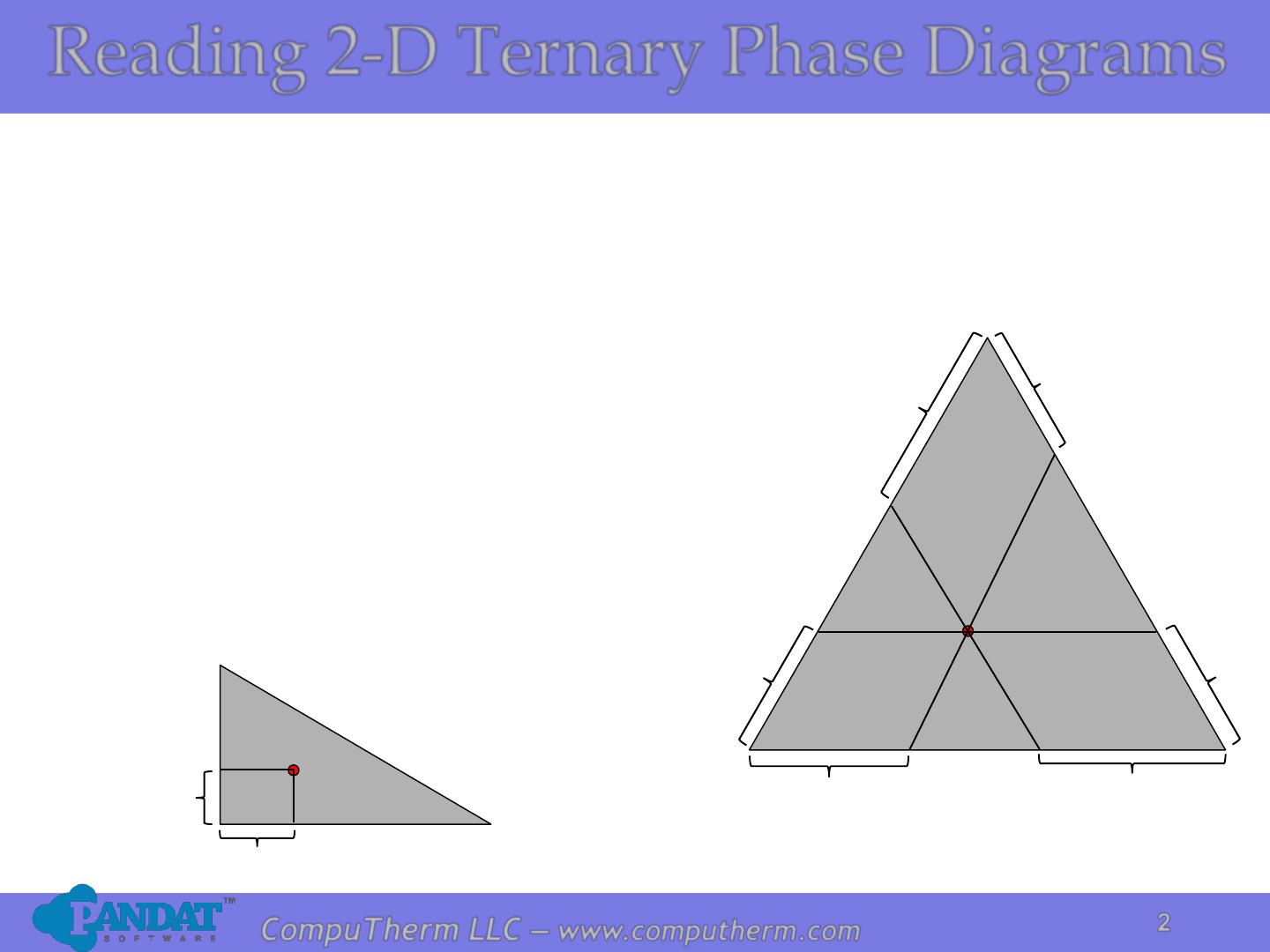
Properties of ternary diagrams. Phase behavior of mixtures containing three components is represented conveniently on a triangular diagram such as those shown in Fig. 1.Such diagrams are based on the property of equilateral triangles that the sum of the perpendicular distances from any point to each side of the diagram is a constant equal to the length of any of the sides.
Ternary or triangular phase diagrams can be used to plot the phase behavior of systems consisting of three components by outlining the composition regions on the plot where different phases exist. Ternary phase diagrams lesley cornish. In figure 57c point m is a. By the so called inverse lever arm rule as shown in the figure 57c.
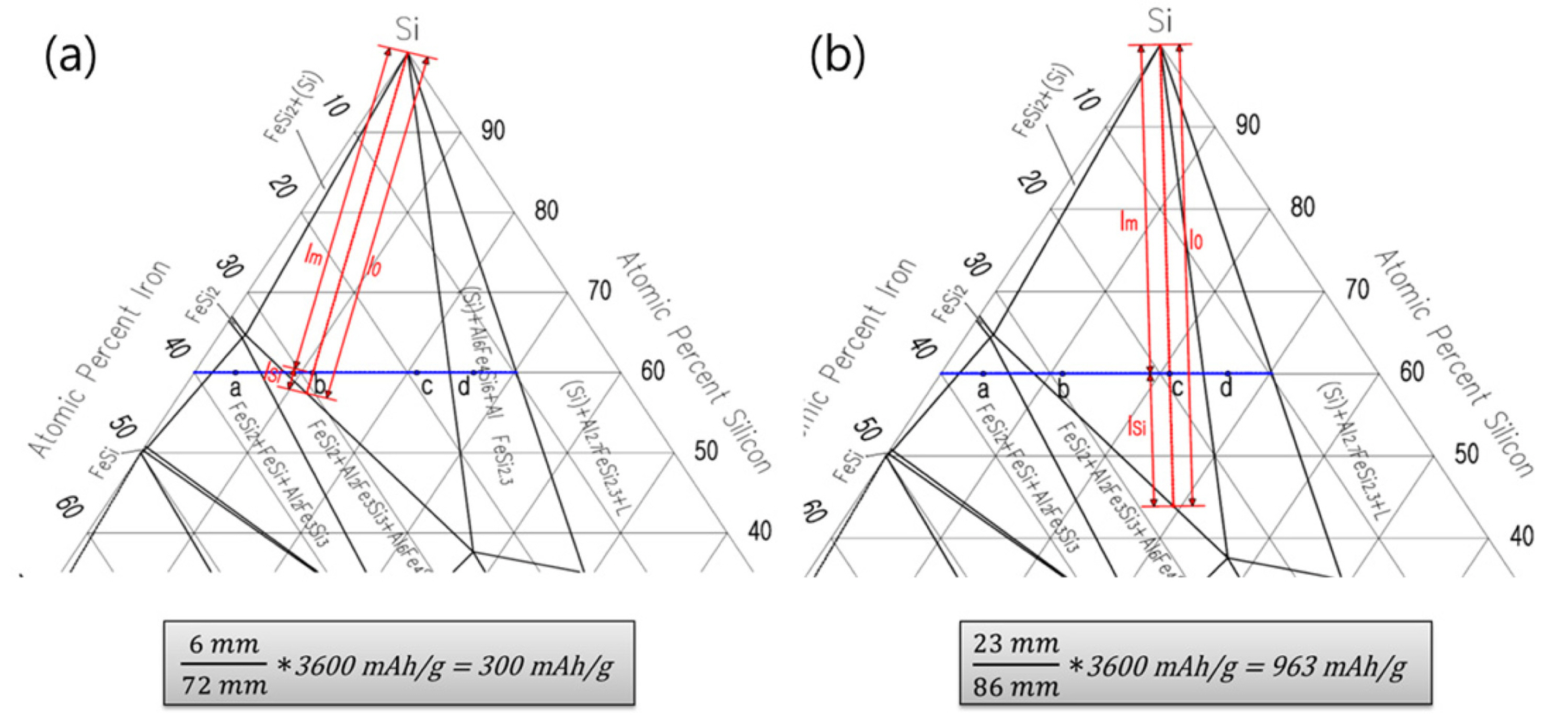
ternary phase diagram of Ni-Cr-Fe. MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 11 Isomorphous system - complete solid solubility of the two components (both in the liquid and solid phases). ... The lever rule is a mechanical analogy to the mass balance
The Gibbs Phase Rule for 3- Components Systems Information regarding phase equilibria can be predicted by a simple rule: F=C+2-P C is the number of components P is the number of phases present F is the degrees of freedom or variance Examples: For pure gas: C = 1 (only one component) and P = 1 (only one phase; the gas phase) F=1+2-1=2 This ...
Uses a simulation to show how ternary phase diagrams are interpreted. This is for a single phase, three component system. The simulation is available at: ht...
Lever rule. In chemistry, the lever rule is a formula used to determine the mole fraction ( xi) or the mass fraction ( wi) of each phase of a binary equilibrium phase diagram. It can be used to determine the fraction of liquid and solid phases for a given binary composition and temperature that is between the liquidus and solidus line.
PHASE COMPOSITION AND LEVER RULE Lever rule: the fractional amounts of two phases are inversely proportional to their distances along the tie line (isotherm) from the bulk composition axis X f Y f L f L 1 1 2 2 L 1 = Xf L 2 = fY 1 1 2 2 1 1 1 f f L f f L 2 1 12 fY XY L f LL 1 Phase 2 "balance the teeter-totter" 459
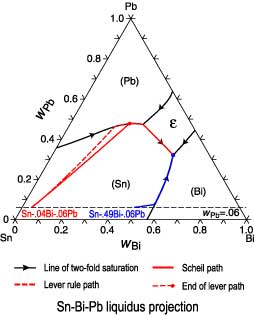
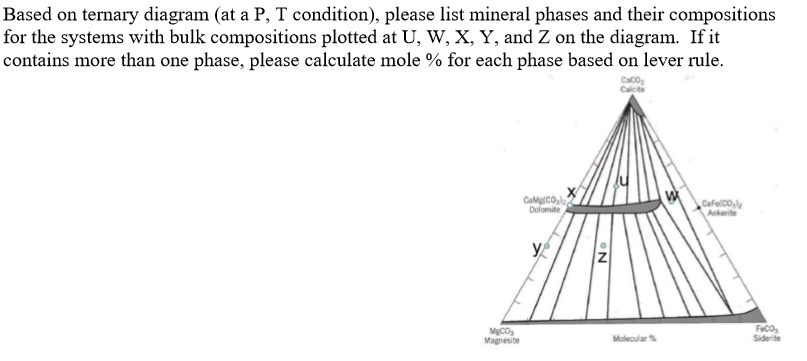
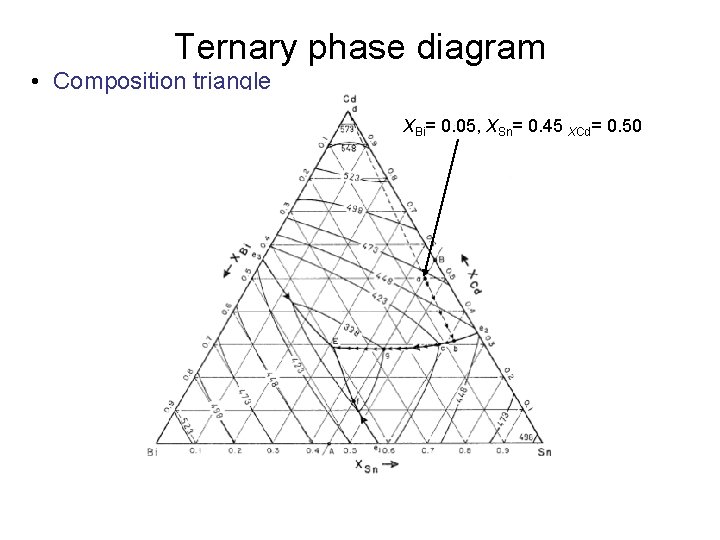
I have read that to know the composition of different phases in a ternary phase diagram of metals A,B,C (at a particular temp. T), we can apply lever rule along the tie line.
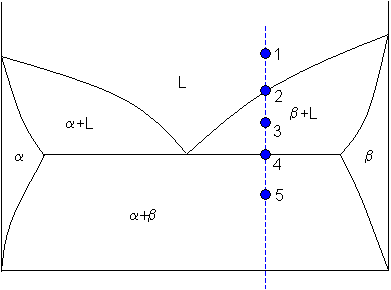
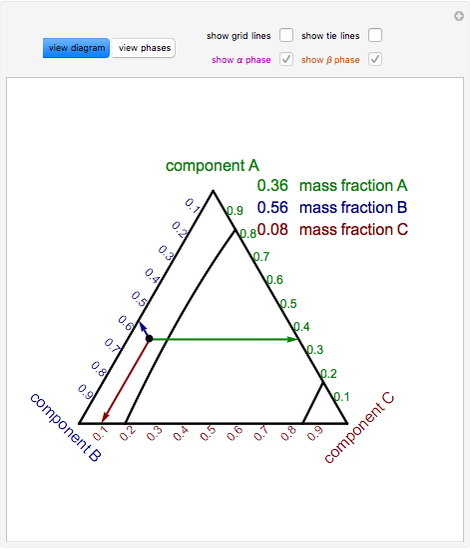
Figure 1. A hypothetical binary phase diagram with an isopleth at 32 weight percent B. Figure 2. A hypothetical ternary phase diagram with an isopleth at 20% A, 70%B, 10%C. When studying phase diagrams, the most common exercise is an isoplethal analysis. An isopleth* is a line of constant composition, shown in Figures 1 and 2. Isopleths are ...
The lever rule is used to calculate the relative amounts of the two phases, which are represented on the bar graph on the right. The compositions of the two phases are indicated by dotted lines on the phase diagram and numerically at the top of the bar graph. Outside the curve, and mix spontaneously to form one layer.
Chapter 10: Ternary Phase Diagrams / 195. Fig. 10.5 . Isopleth through hypothetical ternary phase diagram at a constant . 40% . C. adapted from ref 10.1. SPB Line. The single-phase boundary line is found on any section that . contains a single-phase region. The line is what its name implies. It is the boundary line around that single-phase region.
50 Fresh s How to Read Ternary Phase Diagram. ternary plot a ternary plot ternary graph the first method is an estimation based upon the phase diagram grid the concentration of each species is. Applying the Phase Rule to Ternary Diagrams. ternary diagram basics ternary diagram basics engineer clearly loading mod 01 lec 25 ternary phase diagram ...
Lever arm rule ternary diagram. Figure 1 gives a 3d view of the ternary phase diagram of this system. Like binary phase diagram you could use lever rule to find the amounts of liquid and solid in a two phase field if the composition of the alloy is known. Ternary phase diagrams lesley cornish. Ternary systems are those having three components.
The lever rule is a rule used to determine the mole fraction (x i) or the mass fraction (w i) of each phase of a binary equilibrium phase diagram. It can be used to determine the fraction of liquid and solid phases for a given binary composition and temperature that is between the liquidus and solidus line.













0 Response to "42 lever rule ternary phase diagram"
Post a Comment