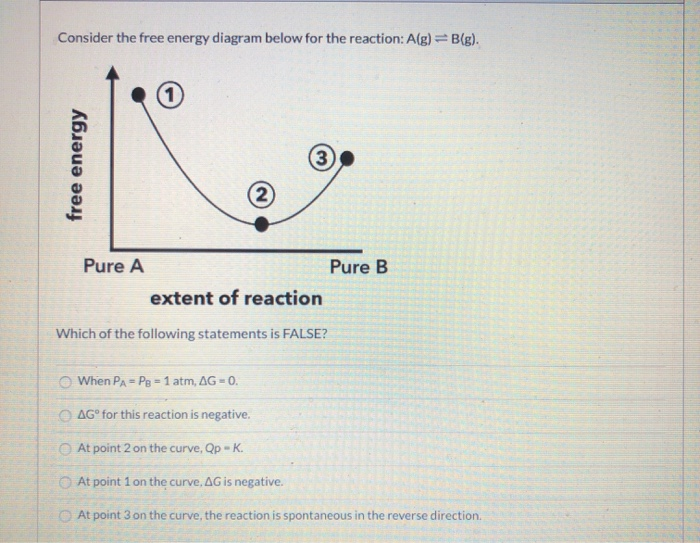
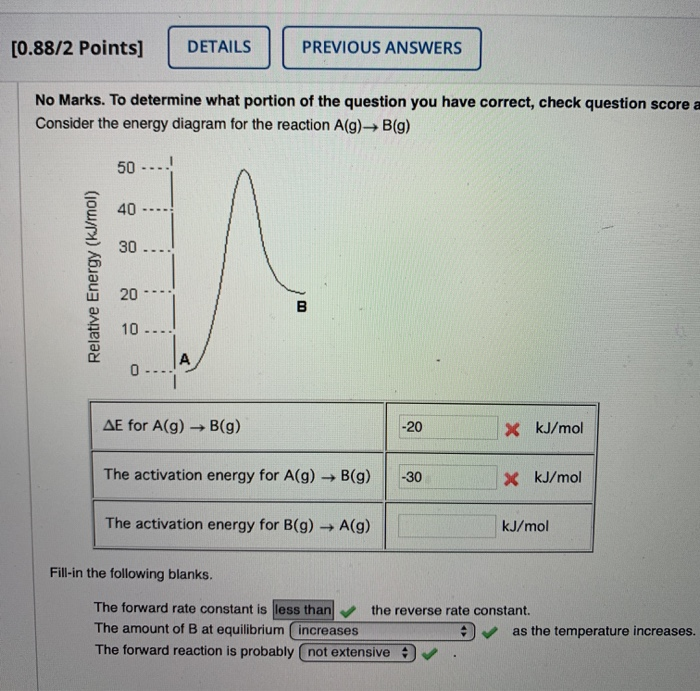
41 consider the energy diagram for the reaction a(g) b(g)
Transcribed Image Text. Consider the Energy diagram below. What does the variable "E" indicate? TS 2 E TS 1 e r g y U Reaction Progress energy of activation S -----> T energy of activation U -----> S free energy change U -----> S free energy change S ----> T. check_circle. Consider the following reaction: A + 3 B → 2 C + 2 D a) Express the rate of this reaction in terms of concentrations of A, B, C and D. ... enthalpy of -23 kJ/mol. Sketch a potential energy diagram for this reaction and determine the activation energy for the reverse reaction? ... The reaction 2 NO (g) + O 2 (g) 2 NO 2 (g) was studied and ...
Consider the energy diagram below. A graph with Reaction Progression on the x axis and Energy on the y axis. A line starts low on the y axis; this section runs horizontally and is labeled A. It rises sharply to a high round peak labeled C.

Consider the energy diagram for the reaction a(g) b(g)
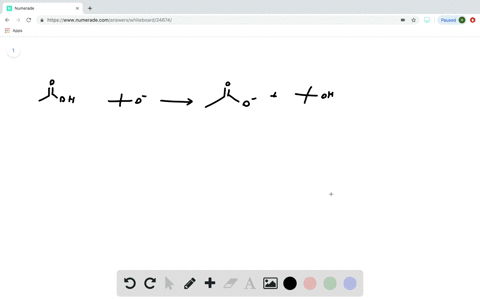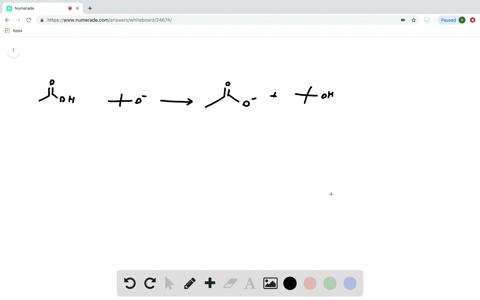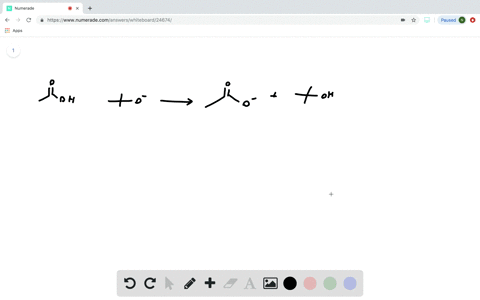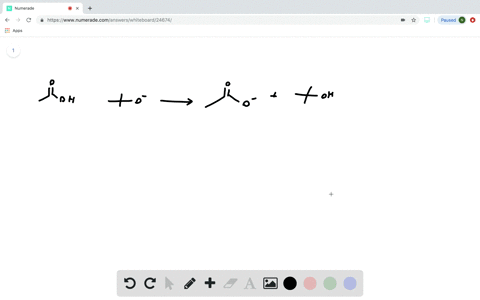
Transcribed image text: Consider the energy diagram for the reaction A(g) rightarrow B(g) Delta E for A(g) rightarrow B(g) 30 kJ/mol The activation energy for A(g) rightarrow B(g) 20 kJ/mol The activation energy for B(g) rightarrow A(g) 50 kJ/mol Apr 9, 2018. Since heat is released for. C3H8(g) + 5O2(g) → 3CO2(g) +4H2O(g) + 2219.9 kJ, we say that ΔH ∘ C = − 2219.9 kJ/mol propane. We approximate that this is the change in potential energy for the reactants going to the products. The above is for an endothermic reaction. A certain feature of combustion reactions suggests that you ... The reaction NO2 (g) + CO (g) → NO (g) + CO2 (g) has ΔH°overall = -226 kJ/mol. A proposed reaction mechanism is shown. Choose the statement (s) that accurately describe the reaction energy diagram for the above reaction. -There will be three peaks. -The Ea of the first step will be larger than the second or third step.
Consider the energy diagram for the reaction a(g) b(g). Consider the potential energy diagram shown below for the reversible reaction R ⎯⎯→ ⎯⎯ P. Which of the following statements is false? A. The reaction mechanism involves three transition states and two intermediates. B. The reaction is endothermic in the forward direction. C. The step X → Y is the rate determining step.. D. Consider the reaction C6H14(ℓ)+ 9.5O2(g) → 6CO2(g)+ 7H2O(ℓ) at constant pressure. Which response is true? ... Refer to the potential energy diagram shown below. A B Reaction progress Energy (kJ) 250 300 350 What is the change in enthalpy (∆H) for ... the internal energy of a reaction? 1. The heat capacity of the calorimeter Click here👆to get an answer to your question ️ Consider the following reversible reaction, A(g) + B(g) AB(g) .The activation energy of the backward reaction exceeds that of the forward reaction by 2RT (in J mol^-1 ). If the pre - exponential factor of the forward reaction is 4 times that of the reverse reaction, the absolute value of Δ G^∘ (in J mol ^-1) for the reaction at 300 K is ... On the axes below, draw a potential energy diagram for the reaction represented by this equation. 19. Given the reaction at equilibrium: A(g)+B(g)+heat (+ C(g)+D(g) The equilibrium will shift to the right when the A. pressure is decreased B. temperature is increased C. concentration of A(g) is decreased D. concentration of C(g) is increased 20.
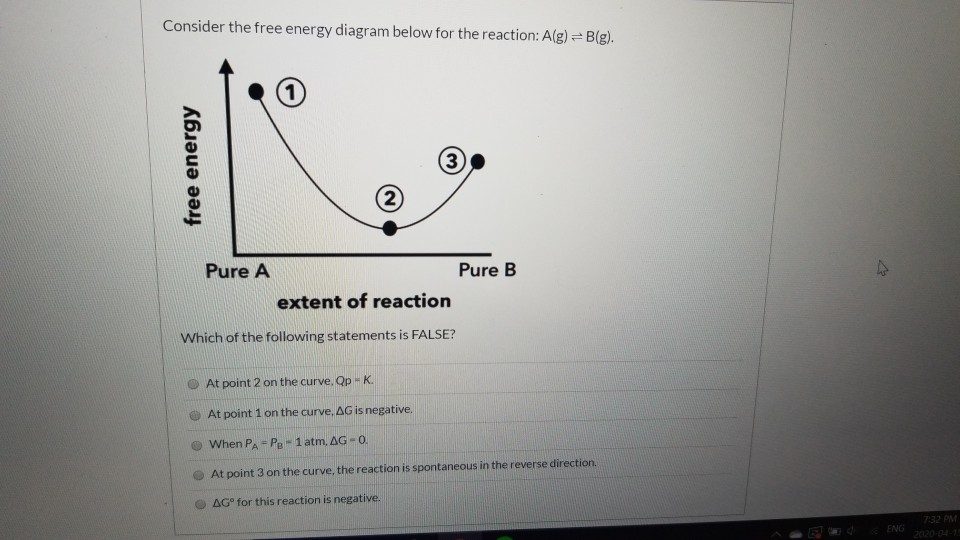
The diagram shows the free energy change of the reaction. A (g) + B (g) ⇌ AB (g) The reaction progress starts on the left with pure reactants, A and B, at 1 atm and moves to pure product, AB, also at 1 atm on the right. Select the true statements. A. The "x" on the graph corresponds to ΔG of this reaction. B. Select the true statement concerning the above potential energy diagram. A. The catalyzed reaction has a larger H. B. The uncatalyzed reaction has a larger H. C. The catalyzed reaction has a greater rate of reaction. D. ... Consider the following reaction: O(g)+ O3(g) --> 2O2(g) 4. Consider the following potential energy diagram The Activation energy for the forward reaction is A. 25 kJ B. 50 kJ C. 75 kJ D. 125 kJ 5. Consider the following reaction: ½ H 2(g) + ½ I 2(g) ----- > HI (g) The activation energy for the formation of HI is 167 kJ and for the decomposition of HI is 139 kJ. The Consider the energy diagram for a solution formed between molecules A and B. What can be said about this solution? D) The solution formed is ideal. ... Consider the reaction: N₂ (g) + 3 Br₂ (g) ⇌ 2 NBr₃ (g) At equilibrium, the concentrations of N₂ and Br₂ are 0.34 M and 0.70 M respectively, and the concentration of NBr₃ is 0.090 M
A) can be called entropy change. B) can be called heat of reaction. C) has a negative value for an exothermic reaction. D) represents the difference between the energy used in breaking bonds and the energy released. in forming bonds in a chemical reaction. E) can be called enthalpy change. can be called entropy change. On the axis below, draw a potential energy diagram for the reaction. Label the reactants and the products on your graph. [Do not number the axis.] Draw an arrow on your Reaction Coordi nate gram to represent the heat of the reaction. Label the arrow AH. The potential energy diagram of a chemical reaction is shown below. 200 - 150 100 - 50 5 The Overall Order of a reaction is the sum of the individual orders: Rate (Ms−1) = k[A][B]1/2[C]2 Overall order: 1 + ½ + 2 = 3.5 = 7/2 or seven−halves order note: when the order of a reaction is 1 (first order) no exponent is written. Units for the rate constant: The units of a rate constant will change depending upon the overall A. It changes the ΔH of a reaction. B. It increases the kinetic energy of the reactants. C. It decreases the potential energy of the products. D. It provides a reaction mechanism with a lower activation energy. 10. Consider the following reaction involving 1.0 g of powdered zinc: Zn (s) + 2HCl (aq) → ZnCl 2(aq) + H 2(g)

Which Of The Following Energy Diagrams Is Of A Reaction With One Transition State Which Of The Following Energy Diagrams Is Of A Reaction With One Intermediate Image Src Reaction39466578977983968 Study Com
The diagram represents an equilibrium mixture for a reaction of the type B(g) + C(g) <---> BC(g). Determine the magnitude of K for the reaction. Assume each molecule or atom represents 1 mole of the species and that the volume of the container is 1 L.
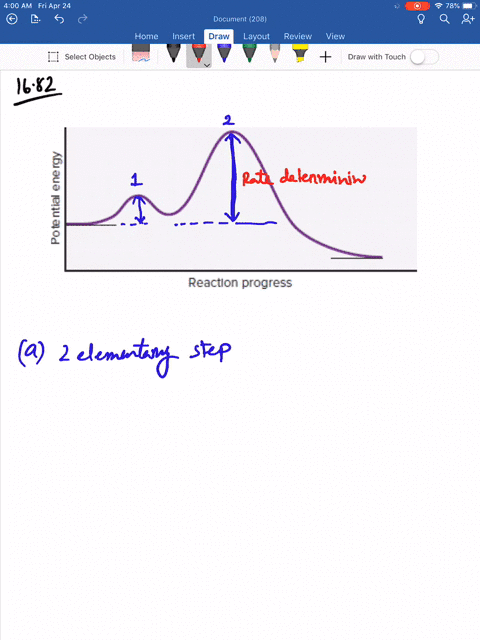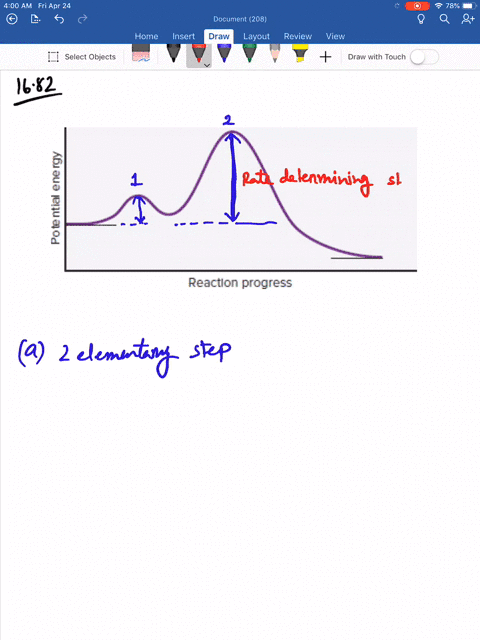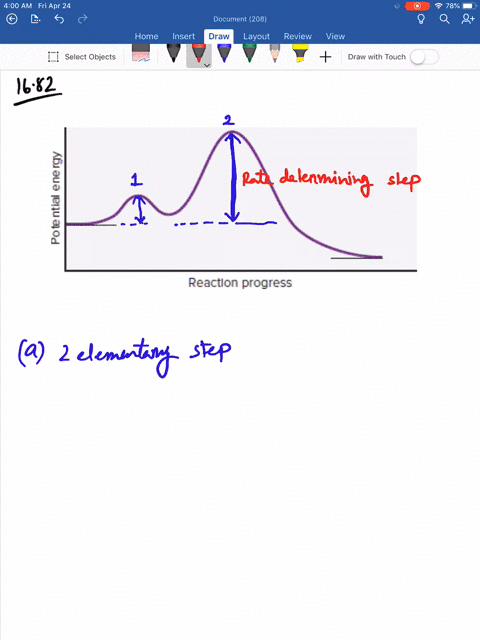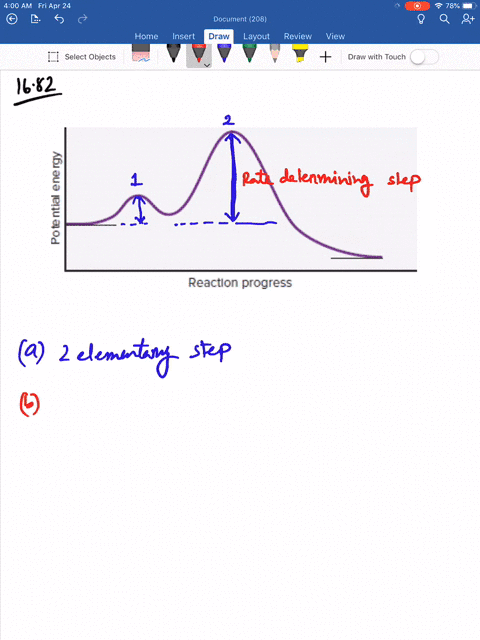
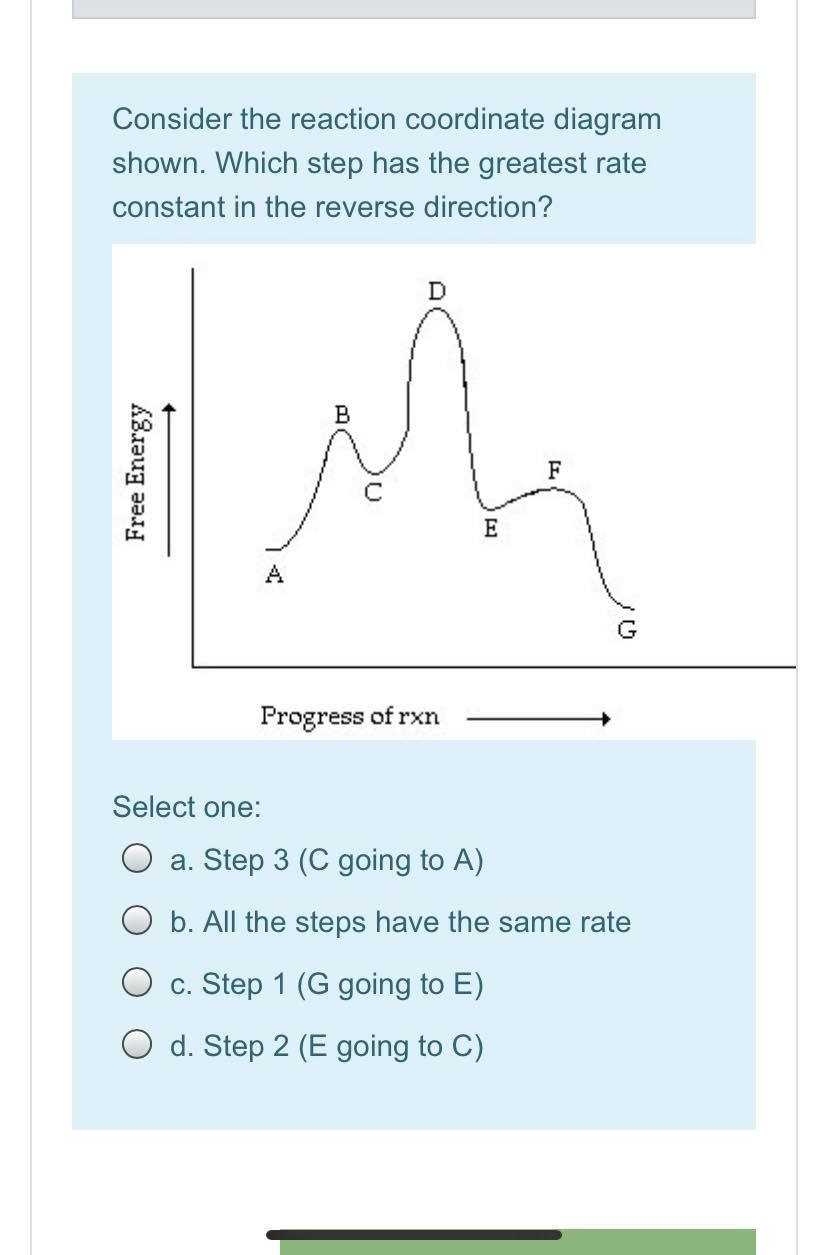
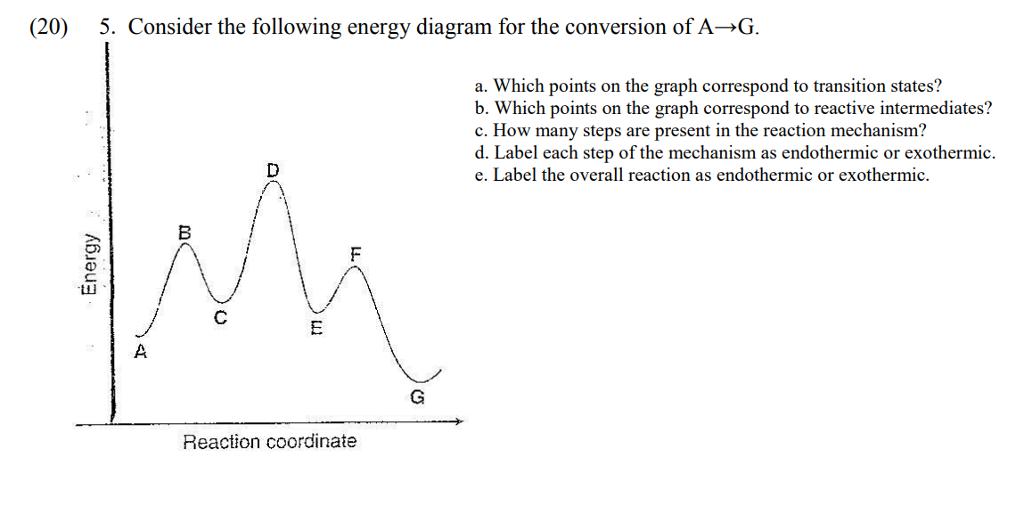
Solved Consider The Following Energy Diagram For The Conversion Of Textbf A Rightarrow Textbf G A Which Points On The Graph Correspond To Transition States B Which Points On The Graph Correspond To Reactive Intermediates
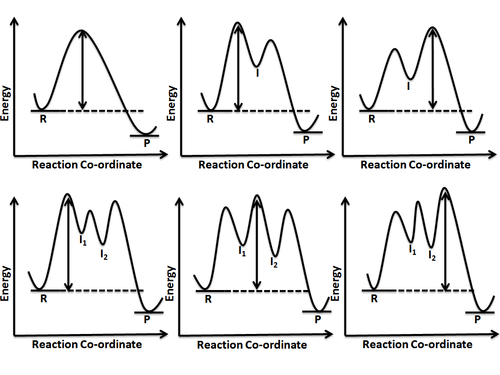
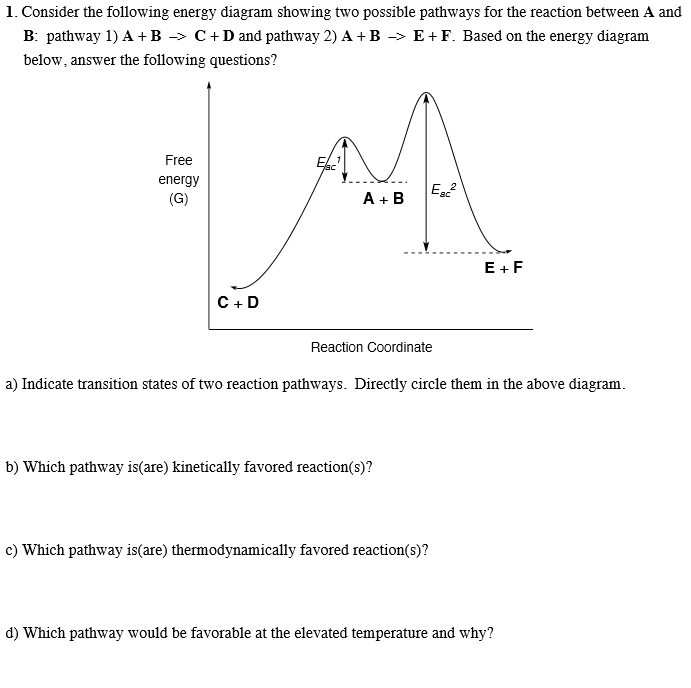
Consider the reaction in which HCl adds across the double bond of ethene: The following mechanism, with the accompanying energy diagram, has been suggested for this reaction: a. Based on the energy diagram, determine which step is rate limiting. b. What is the expected order of the reaction based on the proposed mechanism? c.
Consider the following diagrams which show the progress for the reaction A (blue) ⇌ B (red). The equilibrium constant (K) for this reaction is 0.8. At which point does the reaction reach equilibrium? The equilibrium constant is the products divided by the reactants. A K value of 0.8 is consistent with image choice C where the ratio of product ...

Investigating First Year Undergraduate Chemistry Students Reasoning With Reaction Coordinate Diagrams When Choosing Among Particulate Level Reaction Mechanisms Chemistry Education Research And Practice Rsc Publishing
b. Draw and label potential energy diagram for the reaction including a molecular structure that could represent an activated complex. The activated complex would show an unstable association of one CH 4(g) molecule and O 2(g) molecule with partial bonds. Check Your Solution The potential energy diagram should match the given information.
A. activation energy. B. energy of reaction. C. entropy of reaction D. reaction mechanism energy 21. An 8.00g piece of magnesium was placed into 6.0 M HCl. After 25 s, 3.50 g of unreacted magnesium remained. The average rate at which magnesium was consumed is A. 0.14 g/s B. 0.18 g/s C. 0.32 g/s D. 4.50 g/s 22.
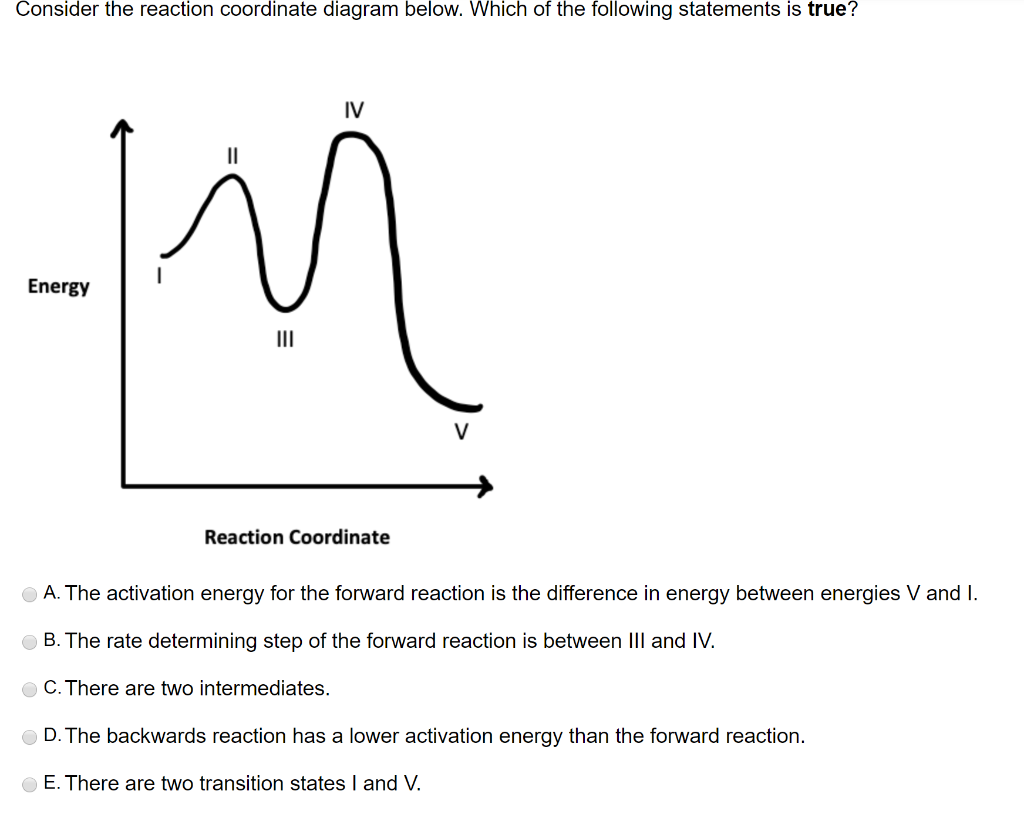
Figure PRS.3B-2 Reaction coordinate for (a) S N2 reaction, and (b) generalized reaction. Collision Theory when discussing the Polyani Equation. The energy barrier shown in Figure PRS.3B-2 is the shallowest barrier along the reaction coordinate. The entire energy diagram for the ABC system is shown in 3-D in Figure PRS.3B-3.
Solved Consider The Following Energy Diagram For The Conversion Of Textbf A Rightarrow Textbf G A Which Points On The Graph Correspond To Transition States B Which Points On The Graph Correspond To Reactive Intermediates
Gibbs free energy (G): the energy available to perform useful work DG < 0 spontaneous DG > 0 nonspontaneous as written, spontaneous in the reverse direction. DG = 0 The reaction is at equilibrium. aA + bB cC + dD DG 0 0 rxn f = [cDG (B)(C) (A)+ dDG f (D) D] - [aDG0 f + b G0 f ] DG0 rxn f = SnDG0 (products)-S mDG0 f (reactants) Standard free ...
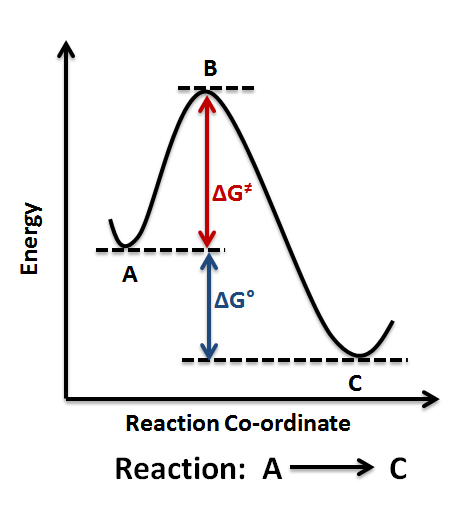
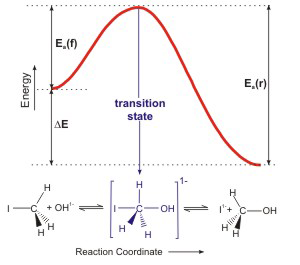
Reaction Coordinate Diagrams Let's consider a general reaction where a reactant or set of reactants, A, is transformed into a product or set of products, B. The diagram below is called a reaction coordinate diagram. It shows how the energy of the system changes during a chemical reaction.
The rate constant for the reaction H 2 (g) + I 2 (g) ---> 2HI(g) is 5.4 x 10-4 M-1 s-1 at 326 o C. At 410 o C the rate constant was found to be 2.8 x 10-2 M-1 s-1. Calculate the a) activation energy and b) high temperature limiting rate constant for this reaction. Answer: All reactions are activated processes.
The reaction NO2 (g) + CO (g) → NO (g) + CO2 (g) has ΔH°overall = -226 kJ/mol. A proposed reaction mechanism is shown. Choose the statement (s) that accurately describe the reaction energy diagram for the above reaction. -There will be three peaks. -The Ea of the first step will be larger than the second or third step.
Apr 9, 2018. Since heat is released for. C3H8(g) + 5O2(g) → 3CO2(g) +4H2O(g) + 2219.9 kJ, we say that ΔH ∘ C = − 2219.9 kJ/mol propane. We approximate that this is the change in potential energy for the reactants going to the products. The above is for an endothermic reaction. A certain feature of combustion reactions suggests that you ...
Transcribed image text: Consider the energy diagram for the reaction A(g) rightarrow B(g) Delta E for A(g) rightarrow B(g) 30 kJ/mol The activation energy for A(g) rightarrow B(g) 20 kJ/mol The activation energy for B(g) rightarrow A(g) 50 kJ/mol
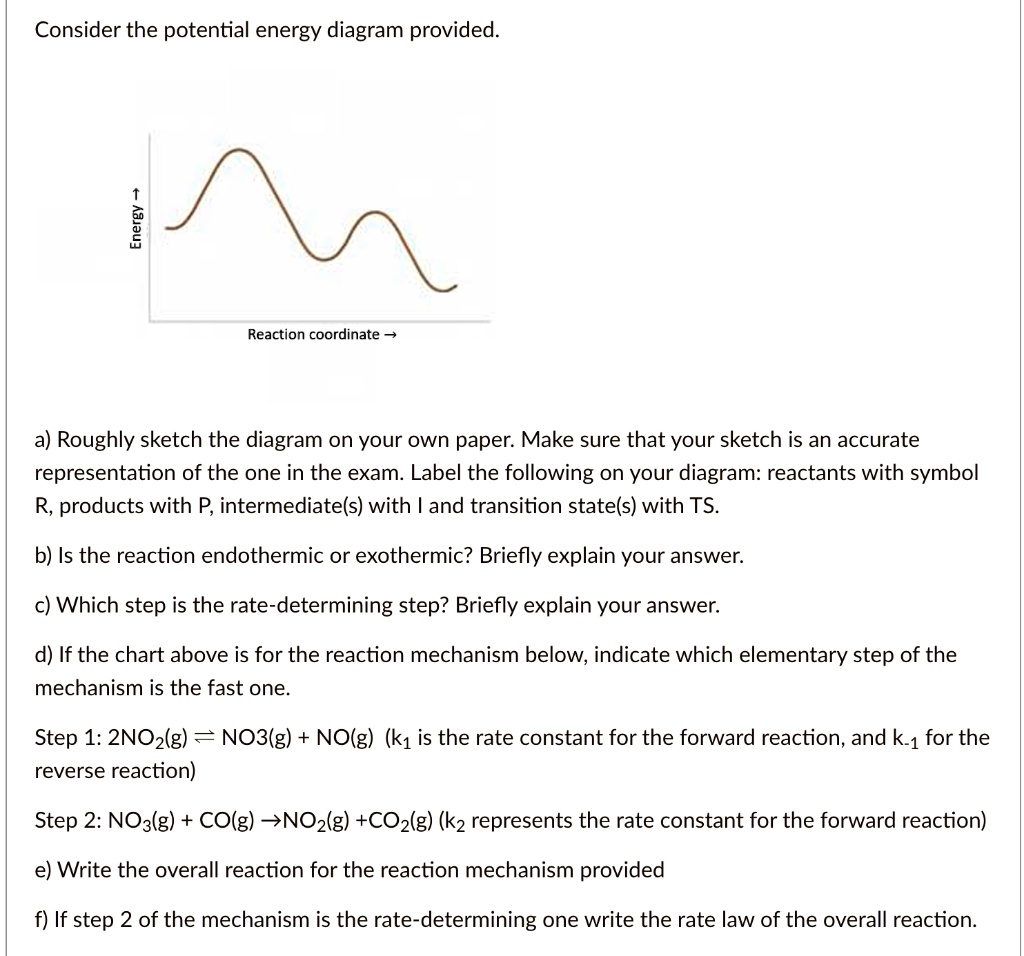
Solved Consider The Potential Energy Diagram Provided 1 Reaction Coordinate A Roughly Sketch The Diagram On Your Own Paper Make Sure That Your Sketch Is An Accurate Representation Of The One In The
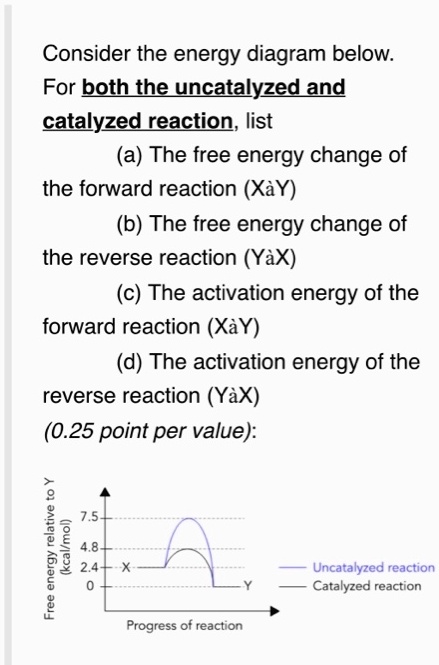
Solved Consider The Energy Diagram Below For Both The Uncatalyzed And Catalyzed Reaction List A The Free Energy Change Of The Forward Reaction Xay B The Free Energy Change Of The Reverse Reaction Yax
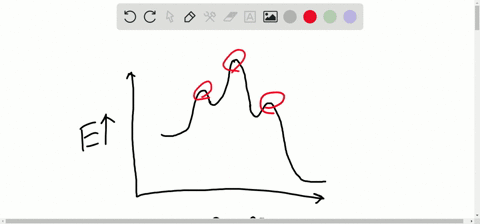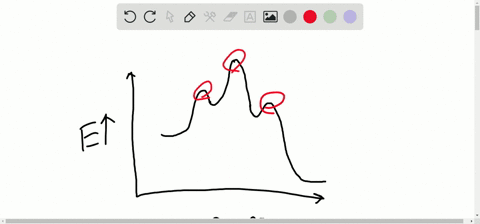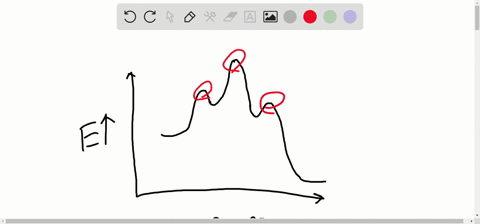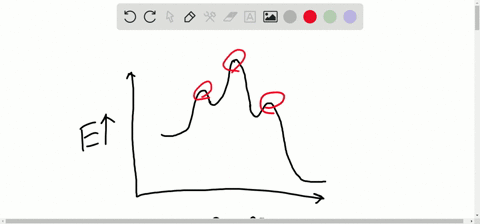
Solved Consider The Following Energy Diagram For The Conversion Of Textbf A Rightarrow Textbf G A Which Points On The Graph Correspond To Transition States B Which Points On The Graph Correspond To Reactive Intermediates

Justify Answers Consider The Energy Diagram Below Showing The Conversion Of A To G A How Homeworklib
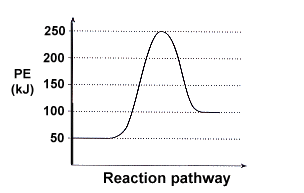
What Is The Activation Energy For The Reverse Reaction In Terms Of The Activation Energy Of The Forward Reaction And The Enthalpy Of Reaction Draw It Out For An Endothermic Reaction


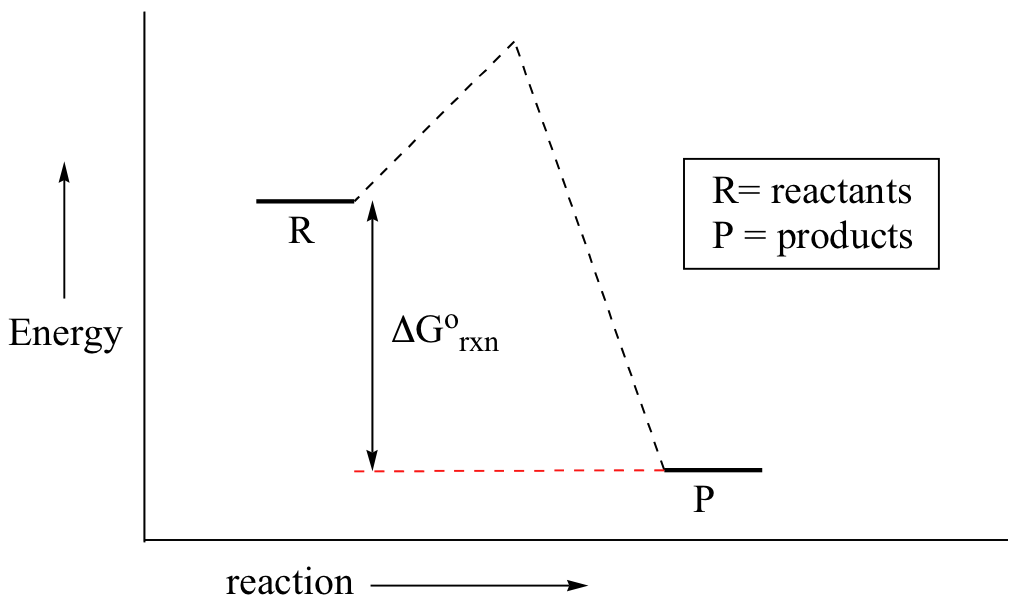















0 Response to "41 consider the energy diagram for the reaction a(g) b(g)"
Post a Comment