39 label the following reaction coordinate diagram
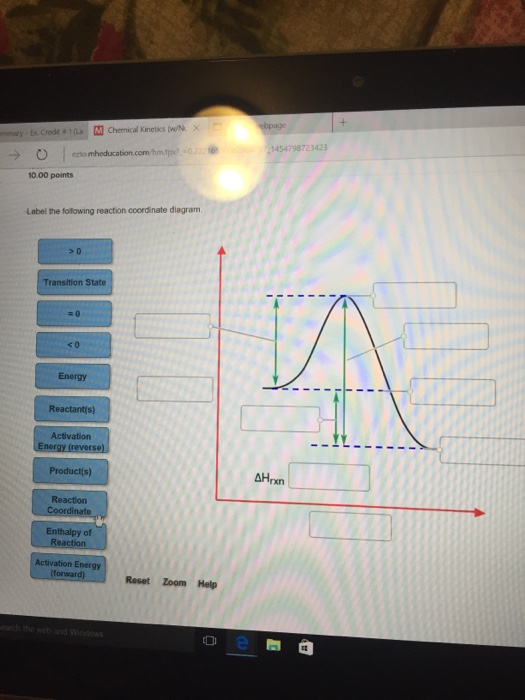
Label the following reaction coordinate diagram. enthalpy of reaction, reactant (s), reaction coordinate, activation energy (reverse), transition state, < 0, > 0, activation energy (forward), = 0, product (s), energy. Our mission is to help you succeed in your Chemistry class. "Clutch really helped me by reinforcing the things I learned in ... Labelthe following reaction coordinate diagram by matching betweenletters and numbers: Answer +20. Watch. 1. answer. 0. watching. 31. views. For unlimited access to Homework Help, a Homework+ subscription is required. Jean Keeling Lv2. 10 Aug 2019. Unlock all answers. Get 1 free homework help answer. Unlock ...
Transition states occur at minima on reaction coordinate diagrams; ... Two possible products can be formed in the following reaction. Label the nucleophile and electrophile in this reaction and then draw the structure for each of the possible products.

Label the following reaction coordinate diagram
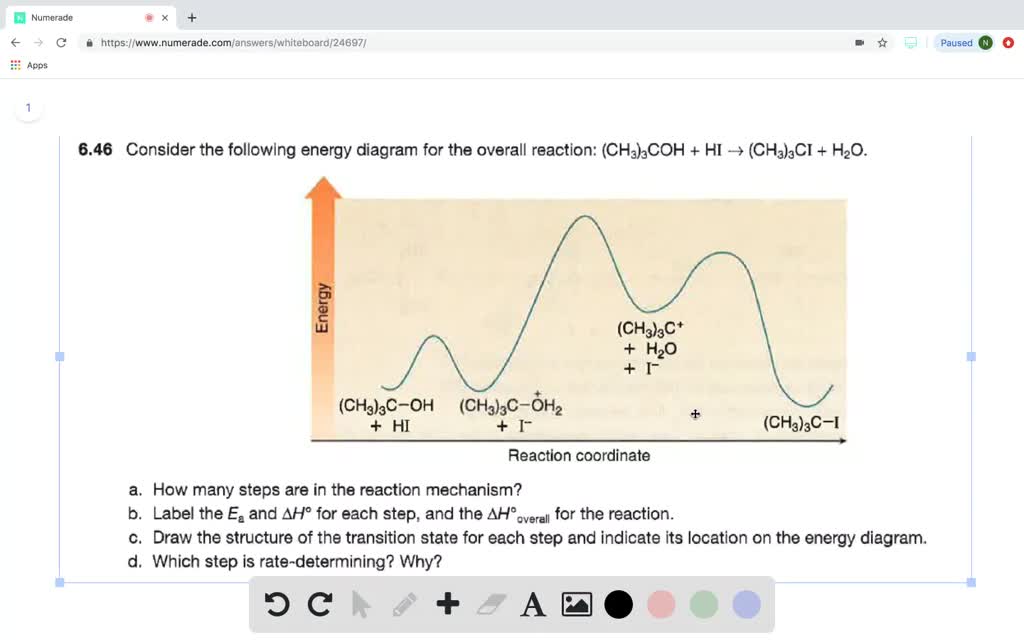
reaction coordinate Br Figure 9.11 Reaction free-energy diagram for the S N1-E1 solvolysis reaction of (CH 3) 3CBr with ethanol.The rate-limiting step,ionization of the alkyl halide (red curve),has the transition state of highest standard free energy.The Solved: Draw a reaction coordinate diagram for a two-step reaction in which the first step is endergonic, the second step is exergonic, and the overall reaction is endergonic. Label the reactants, products, intermediates, and transition states. - Slader The reaction coordinate diagram for the ozone photolysis reaction is a little different from those above because this is an endothermic reaction. Label the following reaction coordinate diagram by matching between letters and numbers. Textbook solutions expert qa. In chemistry a reaction coordinate is an abstract one dimensional coordinate ...
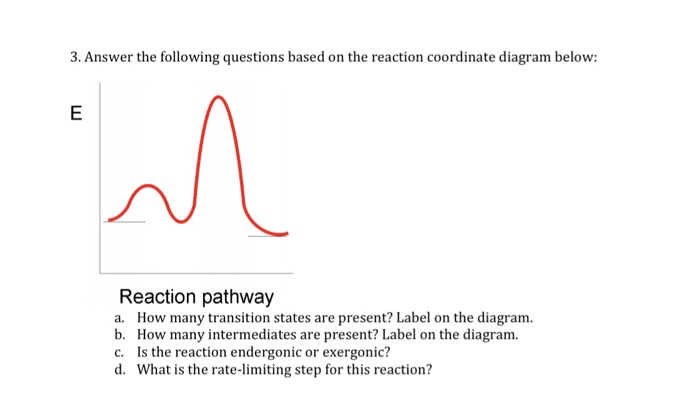
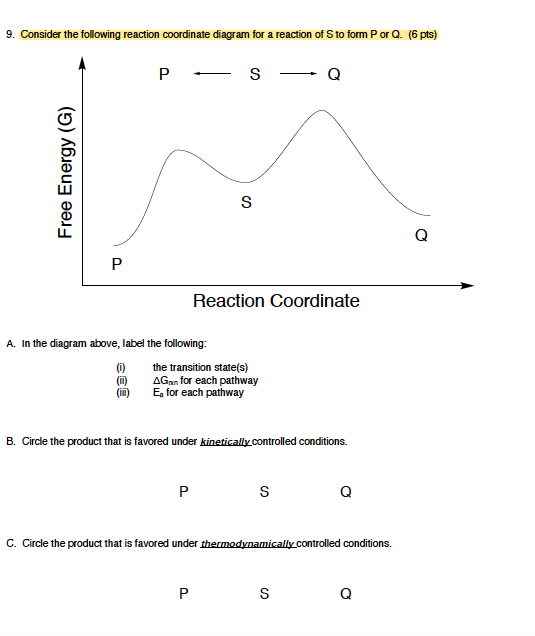
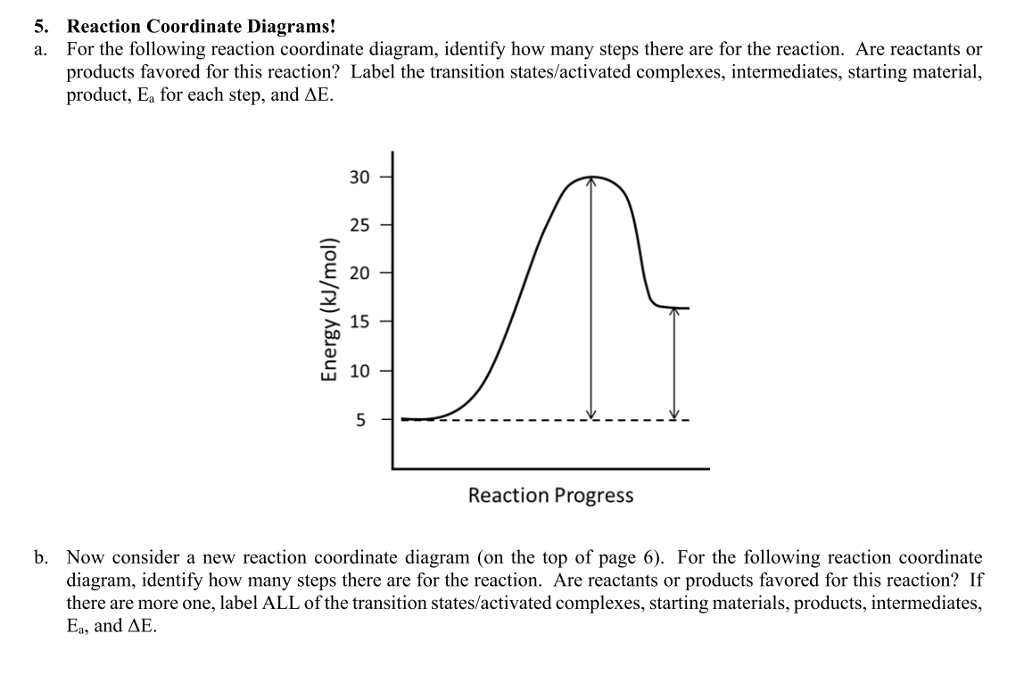
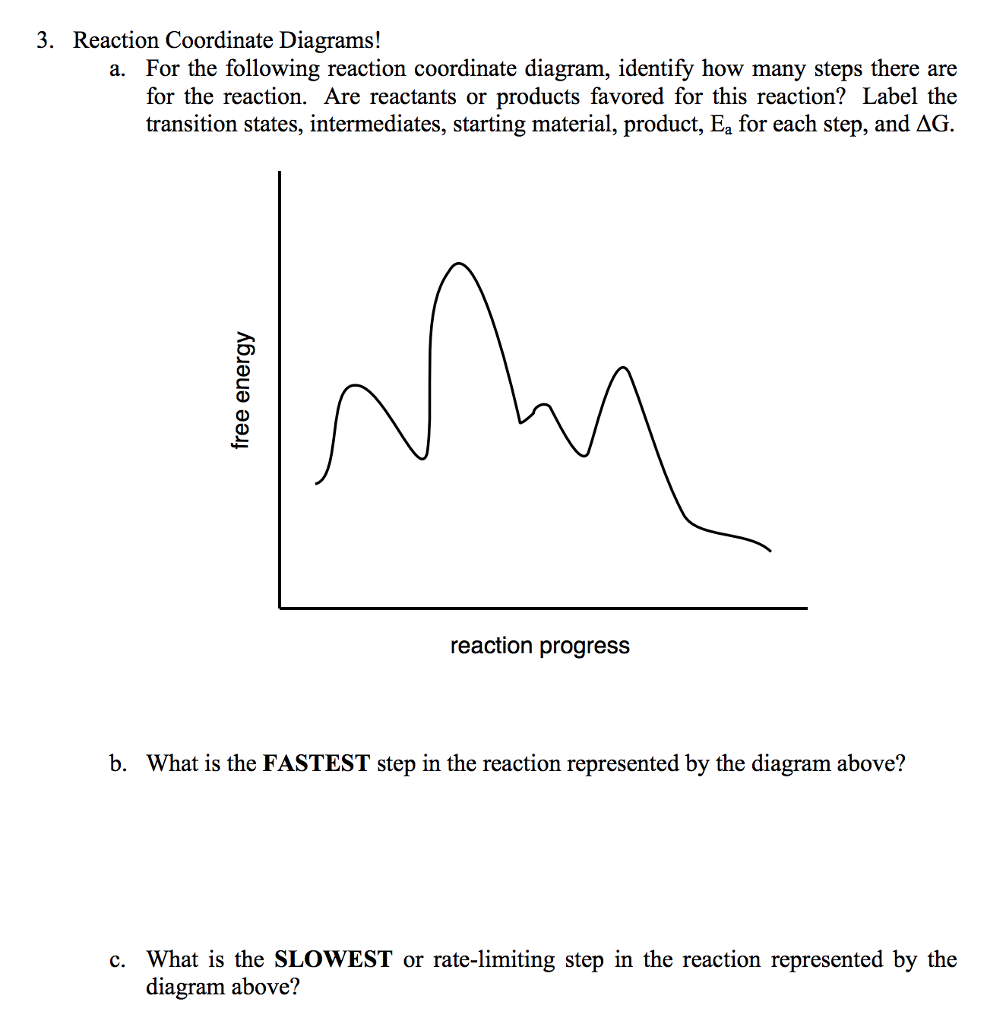
Label the following reaction coordinate diagram. (6 pts) P - SQ Free Energy (G) s Q P Reaction Coordinate A. In the diagram above, label the following: (0) the transition state(s) AG for each pathway E for ... The fully filled in reaction coordinate diagram is displayed below. The arrow marked in the question represents the activation energy, which is the energy barrier that must be overcome in order for the reactants to form products. This reaction is also exothermic because the energy of the products is lower than that of the reactants. Representing a Reaction with a Potential Energy Diagram (Student textbook page 371) 11. Complete the following potential energy diagram by adding the following labels: an appropriate label for the x-axis and y-axis, E a(fwd), E a(rev), ΔH r. a. Is the forward reaction endothermic or exothermic? b. April 20, 2015 - For the following reaction coordinate diagram idemity low many steps there are soe de seaction are rcactants or products favorod for...
HomeworkLib.com is a free homework help website. You can ask any homework questions and get free help from tutors. Typically, we envision reactions proceeding left to right along the reaction coordinate, so often, the activation energy is only noted for the forward reaction. The activation energy on the diagram below shows the barrier to be 102.6 kJ mol -1 . For the reaction coordinate diagram below, label the following: Reactants (R) Activation energy (E.) ΔΗχη Transition State (TS) Energy | A Han Reaction ... Transcribed image text: Homework Saved Label the following reaction coordinate diagram Activation Energy poversa) <0 A Enthalpy of Reaction Product(s) 0 ...
Answer to Label the energy diagram (7 bins) and indicate which reaction corresponds to the energy diagram. The y-axis of the Maxwell-Boltzmann graph can be thought of as giving the number of molecules per unit speed. So, if the graph is higher in a given region. If playback doesn't begin shortly, try restarting your device. A Reaction coordinate Label the following according to he numbers on the figure above. Number of the arrow on the energy diagram Energy of reactants Energy of products AEreaction Ea for the forward reaction Type the correct molecular structure of the activated complex. Energy, E July 21, 2019 - Show transcribed image text. Label the following reaction coordinate diagram by. Solved Label The Following Multi St... Chapter 7. Label the different energies on the following energy diagram. On this graph, the x -axis is the reaction coordinate, while the y -axis is energy. Adding a catalyst to a reaction can stabilize the transition state, thereby reducing the activation energy of that reaction.
May 26, 2019 - Potential energy diagrams chemistry catalyst endothermic exothermic reactions duration. The natural log of a number less than one is a nega...
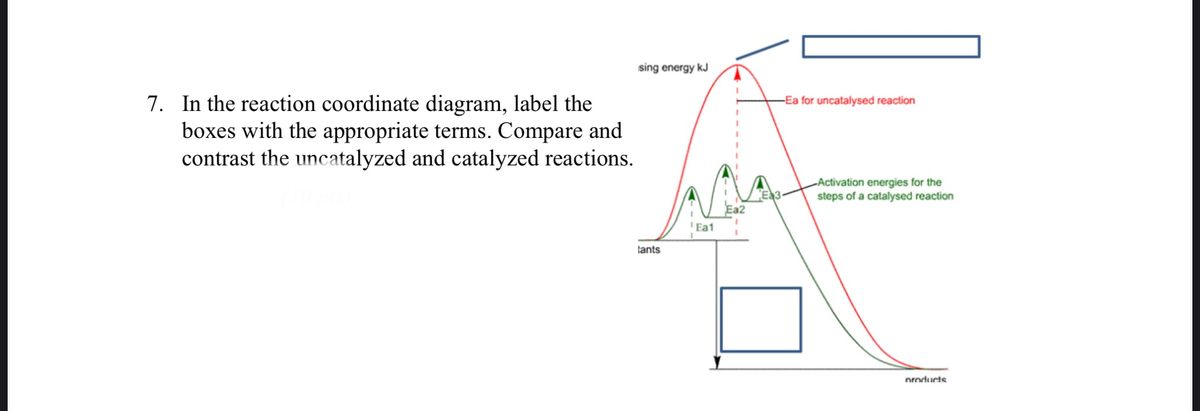
A comparison of the reaction coordinate diagrams (also known as energy diagrams) for catalyzed and uncatalyzed alkene hydrogenation is shown in Figure 1. Figure 1. This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation.
Request unsuccessful. Incapsula incident ID: 273000150284138039-759374793753560013
Energy/Reaction Coordinate! Diagrams! Thermodynamics, Kinetics ! Dr. Ron Rusay" A Reaction Coordinate (Energy) Diagram Thermodynamic Quantities Gibbs standard free energy change (ΔGo) Enthalphy (ΔHo): the heat given off or absorbed during a reaction Entropy (ΔSo): a measure of freedom of motion ΔGo = ΔHo - TΔSo ΔG,ΔH,ΔS, ΔE are state ...
The half-life of a reaction is a. twice as long for a second-order reaction as it is for a first-order reaction b. the time it takes for the amount fo product formed to equal one-half of its initial value c. how long the reaction can run before stopping d. the time it takes for the reactant concentration to decrease to one-half of its initial value
Reaction Coordinate Diagram of Ozone Photolysis The reaction coordinate diagram for the ozone photolysis reaction is a little different from those above because this is an endothermic reaction . Together, the products O 2 and atomic O, have a higher energy than the reactant O 3 and energy must be added to the system for this reaction.
Draw a reaction coordinate diagram for this reaction as above but add the activation energy, E a, for the catalyzed reaction on the appropriate curve in this diagram and label it. This is a bit more subtle since .Types of catalysts (article) | Kinetics | Khan AcademySection The Rate of a Reaction. Search for:
Question: Label the following reaction coordinate diagram. This problem has been solved! See the answer ...
Chemical Engineering Q&A Library a) Draw the reaction coordinate diagram (reaction progress vs. energy) that is consistent with this mechanism. Assume the reaction is exothermic. Label where is ES appears in the diagram. The Michaelis-Menten equation gives the rate law for this mechanism: Rate = k2[E]o[S]/(Ka + [S]) b) With an understanding of this rate law, identify which of the following ...
April 9, 2017 - Energy reactants transition state products activation energy forward transition state activation energy forward energy enthalpy of entha...
HomeworkLib.com is a free homework help website. You can ask any homework questions and get free help from tutors.
Professor Patricia Shapley, University of Illinois, 2012
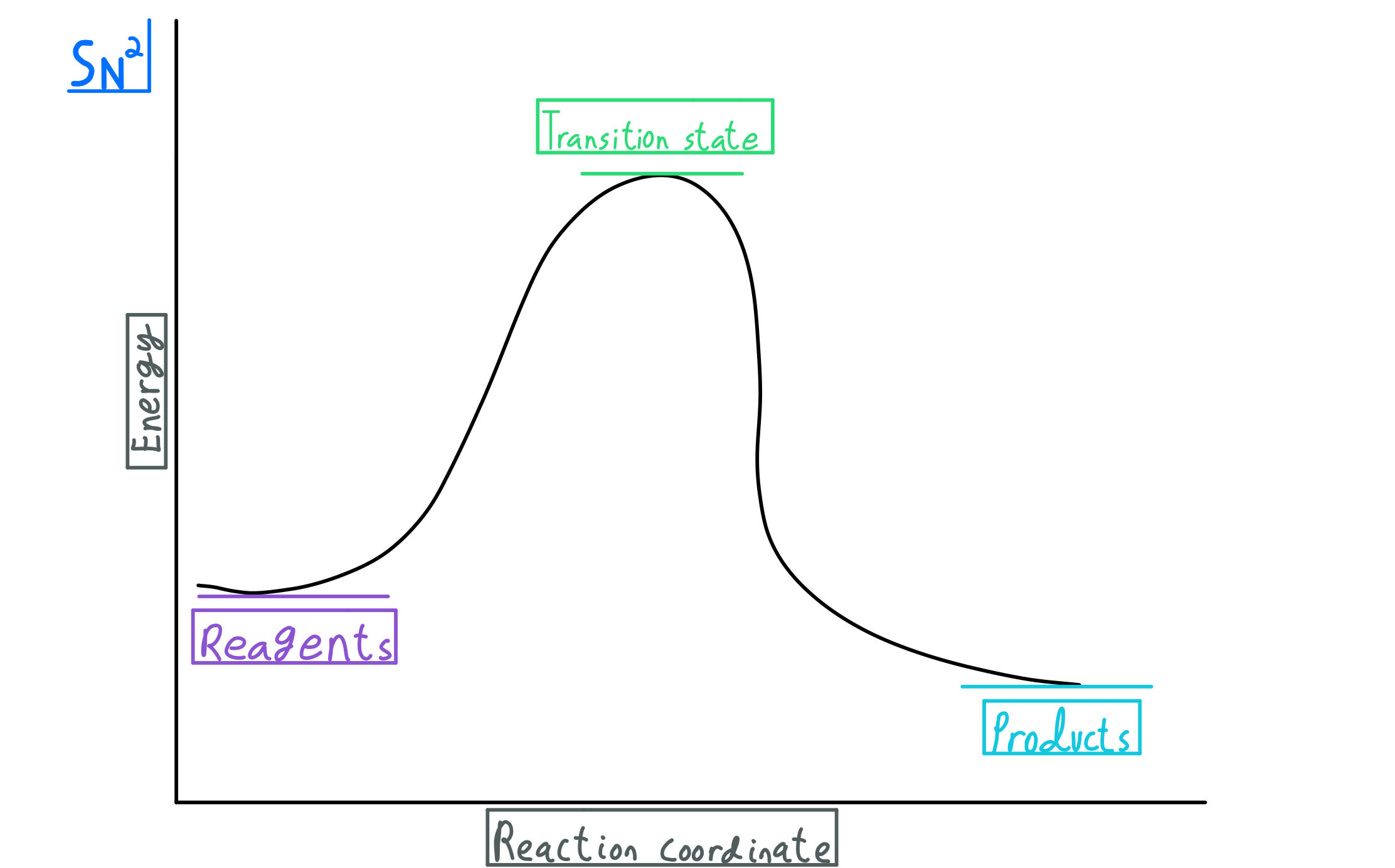
Transcribed image text: Label the following reaction coordinate diagram. <0 >0 Energy Transition State ces Reactant(s) - Activation Energy (forward) 0 ...
Transcribed image text: Chapter 13, Question 44(b) Energy, E Reaction coordinate Label the following according to the numbers on the figure above. Number of the arrow on the energy diagram Energy of reactants Energy of products AE reaction E for the forward reaction Type the correct molecular structure of the activated complex.
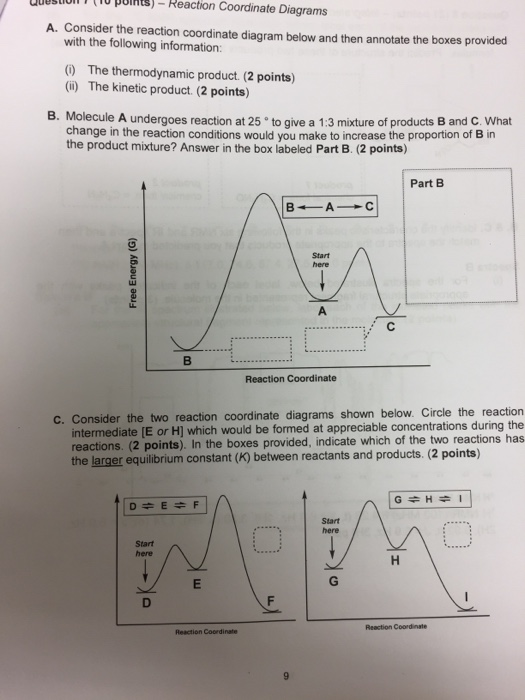
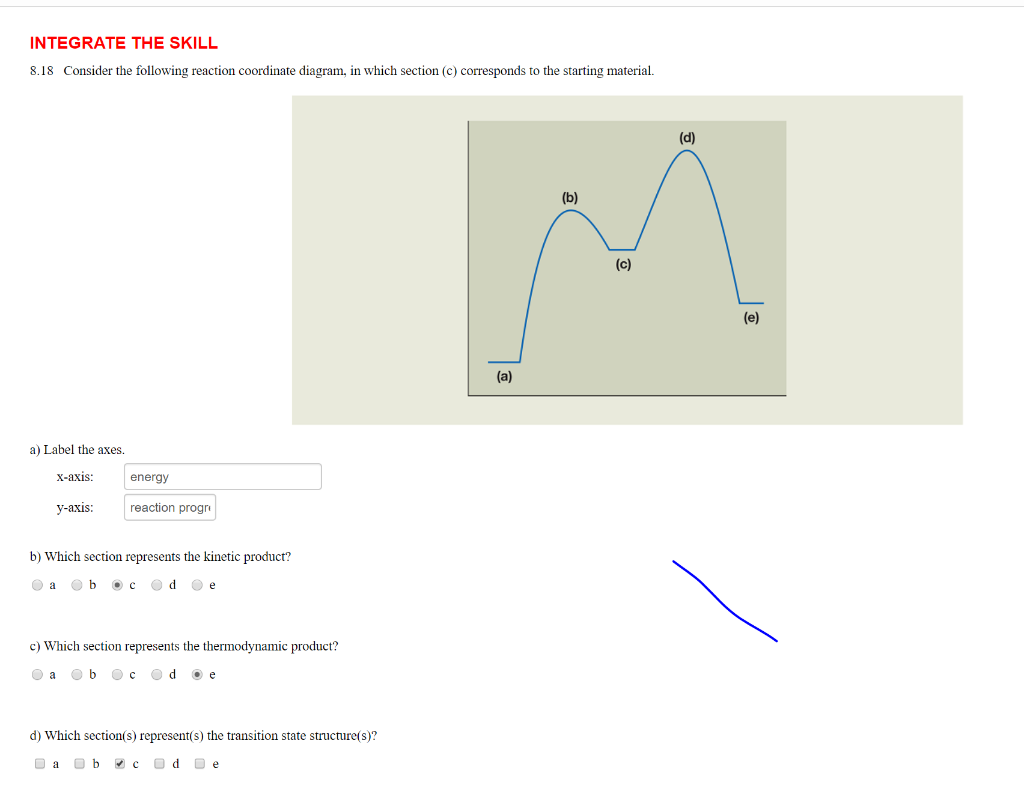
Label the following reaction coordinate diagram by matching between letters and numbers: (diagram in kinetics pt 2 folder in energy diagram folder on desktop) 1- J ... -Label the multi-step reaction energy diagram below using the letters corresponding to the labels on the left. There are more labels than needed; each label can be used only once.
which of the following is a subatomic particle located in the center of the atom and has a positive charge? ... label the diagram below with the different types of electromagnetic radiation in the EM spectrum ... products, and energy changes in the reaction coordinate diagram below. Define the reaction as endothermic or exothermic-reactants ...
Chemistry questions and answers. Label the following reaction coordinate diagram. Energy Reactant (s) Transition State Product (s) Activation Energy (forward) Transition State Activation Energy (forward) Energy Enthalpy of Enthalpy of Reaction Product (s) Reaction AHrxn Reactant (s) Reaction Coordinate Reaction Coordinate Reset Zoom.
Problem Details. Label the following reaction coordinate diagram by matching between letters and numbers: All Chemistry Practice Problems Energy Diagram Practice Problems. Q. A reaction coordinate diagram is shown below for the reaction of A to form E. Answer the following questions.i) Identify the transition state (s)?ii) W...
December 28, 2018 - Label the following reaction energy diagram for a catalyzed and an uncatalyzed process. Uncatalyzed reaction activation energy substrate s catalyzed reaction product p. Please check out the link below. Draw and label a reaction coordinate diagram for an uncatalyzed reaction s to p and a reaction ...
33. Draw the structures of the following functional groups,pH 7.0: (8 pts) (a) hydroxyl (b) carboxylate (c) amino (d) phosphoryl 34. (a) On the reaction coordinate diagram shown below, label the transition state and the overall free-energy change (∆G) for the endergonic (+∆G) reaction? (c) Draw a second curve showing the
Transcribed image text: Given the following reaction coordinate diagram. a. Label the reactants and products in the diagram. (1 pt.) b. Label the transition ...
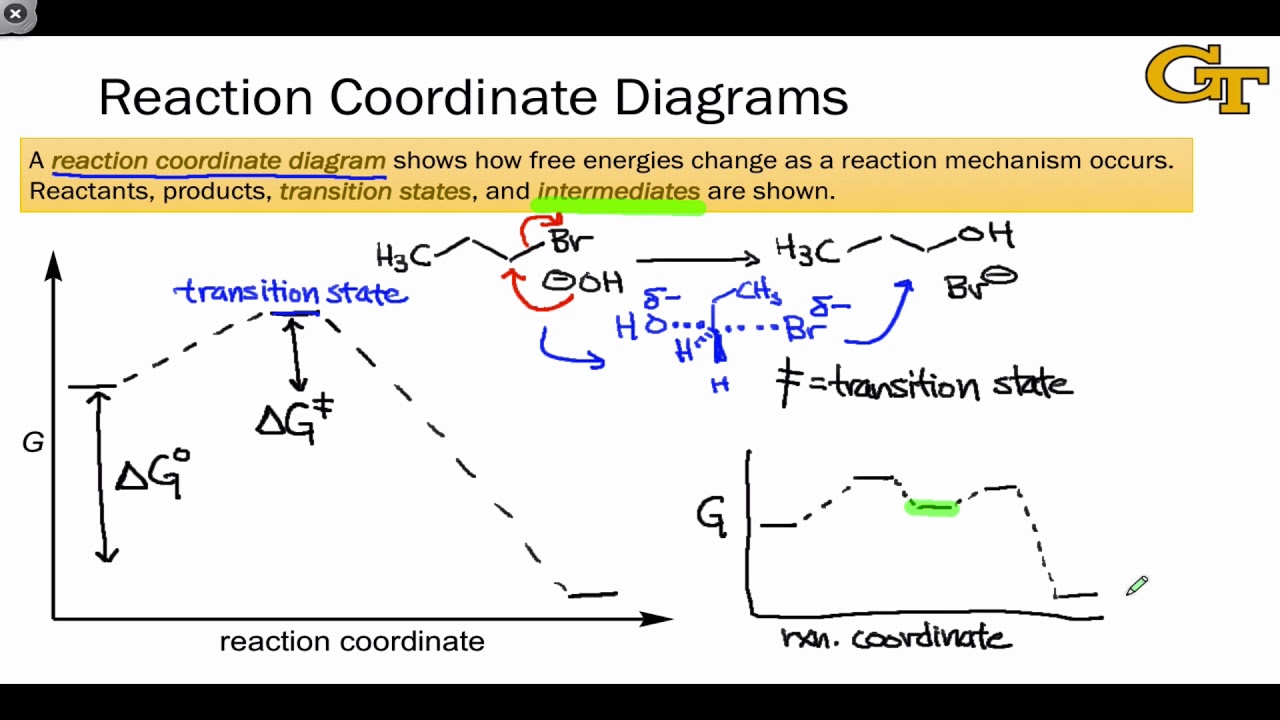
You may recall from general chemistry that it is often convenient to describe chemical reactions with energy diagrams. In an energy diagram, the vertical axis represents the overall energy of the reactants, while the horizontal axis is the 'reaction coordinate', tracing from left to right the progress of the reaction from starting compounds to final products.
Get homework help fast! Search through millions of guided step-by-step solutions or ask for help from our community of subject experts 24/7. Try Study today.
On this diagram we see: the x-axis that is a reaction coordinate: a loosely defined term meaning the reaction progress in the general direction from the starting materials or reagents (SM) to the products (Pr). the energy curve describing the energy states of the components at a certain point in the reaction.
Transcribed image text: Label the following reaction coordinate diagram Enthalpy of Reaction Activation Energy (forward) Reactant(s) Transition State = 0 ...
1. Draw and label a pair of axes. Label the vertical axis "Potential Energy" and the horizontal axis "Reaction Coordinate". 2. Draw and label two short horizontal lines to mark the energies of the reactants and products. 3. Draw the energy level diagram. There must be a hump in the curve to represent the energy level of the activated complex. 4.
You may recall from general chemistry that it is often convenient to describe chemical reactions with energy diagrams. In an energy diagram, the vertical axis represents the overall energy of the reactants, while the horizontal axis is the 'reaction coordinate', tracing from left to right the progress of the reaction from starting compounds to final products.
Label the different energies on the following energy diagram. On this graph, the x-axis is the reaction coordinate, while the y-axis is energy. Adding a catalyst to a reaction can stabilize the transition state, thereby reducing the activation energy of that reaction.
One such reaction is catalytic hydrogenation, the process by which hydrogen is added across an alkene C=C bond to afford the saturated alkane product. A comparison of the reaction coordinate diagrams (also known as energy diagrams) for catalyzed and uncatalyzed alkene hydrogenation is shown in Figure \(\PageIndex{1}\).
Draw a properly labeled reaction coordinate diagram for a reaction with the following. criteria: make sure to clearly indicate Î G and any activation energies (for the forward reaction) as well as all intermediates and transition states. a) exergonic 3 step reaction. b) the first step is the rate-determining step
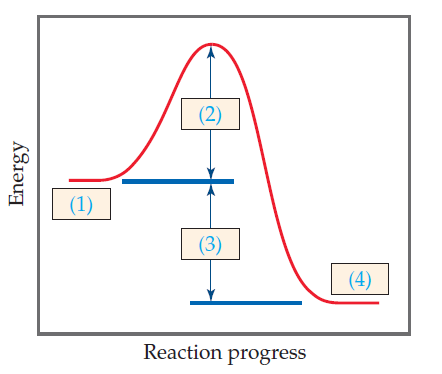
Potential energy Use the labels on the potential energy diagram below (a-f) to answer the following questions. (d) (b) (c) (e) (a) Reaction coordinate (X + Y 2) 1. Which letter on the diagram represents the potential energy of the products?. 2. Which letter indicates the potential energy of the reactants? 3.
July 7, 2020 - Solution for 1. Answer the following questions on the reaction coordinate diagram pictured below. Reaction Progress a. Label AG on the diagram above. b. Label…
The reaction coordinate diagram for the ozone photolysis reaction is a little different from those above because this is an endothermic reaction. Label the following reaction coordinate diagram by matching between letters and numbers. Textbook solutions expert qa. In chemistry a reaction coordinate is an abstract one dimensional coordinate ...
Solved: Draw a reaction coordinate diagram for a two-step reaction in which the first step is endergonic, the second step is exergonic, and the overall reaction is endergonic. Label the reactants, products, intermediates, and transition states. - Slader
reaction coordinate Br Figure 9.11 Reaction free-energy diagram for the S N1-E1 solvolysis reaction of (CH 3) 3CBr with ethanol.The rate-limiting step,ionization of the alkyl halide (red curve),has the transition state of highest standard free energy.The

















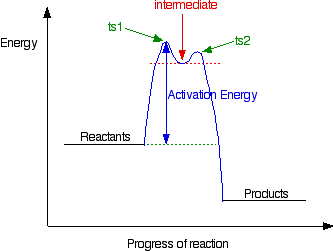
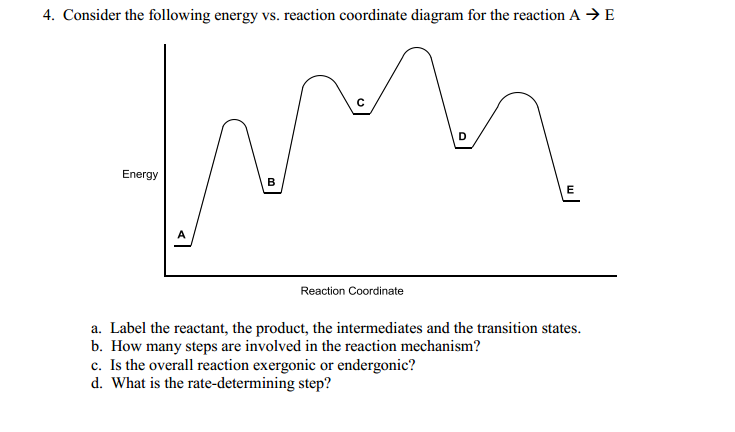
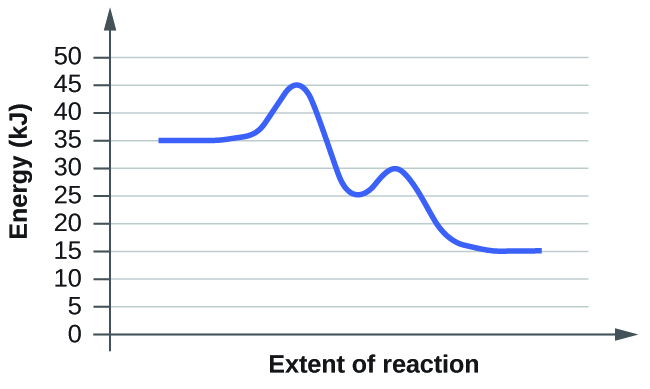

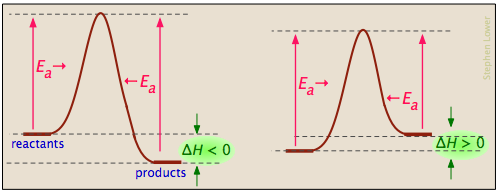
0 Response to "39 label the following reaction coordinate diagram"
Post a Comment