39 ne2+ molecular orbital diagram
Write the molecular orbital structure for the Ne2 (at nr 10) molecule. Does this molecule exist?... ... Not the answer you're looking for? Ask your own homework help question. Our experts will answer your question WITHIN MINUTES for Free. ... Molecular Orbital Model 3. Draw the molecular orbital energy level diagram ... Answer to For Ne2, construct three molecular orbital diagrams, one each for the neutral molecule, the +1 cation, and the -1 anion. According to Molecular Orbital theory, only those molecule can exists which have net positive bond order while the molecules with negative or.
Problem: Determine the bond order from the molecular orbital diagram of Ne2. Does the bond order calculated agree with what you would draw for Lewis ...
Ne2+ molecular orbital diagram
March 4, 2018 - Brainly.in is a part of the largest social network for studying in a group. We provide the best tools for mutual help with school subjects. Join us! With the help of molecular orbital diagram show that Ne2 cannot exist as stable molecule. (Atomic number of Ne = 10). asked Jan 26, 2020 in Chemistry by SurajKumar ( 66.2k points) In order to continue enjoying our site, we ask that you confirm your identity as a human. Thank you very much for your cooperation
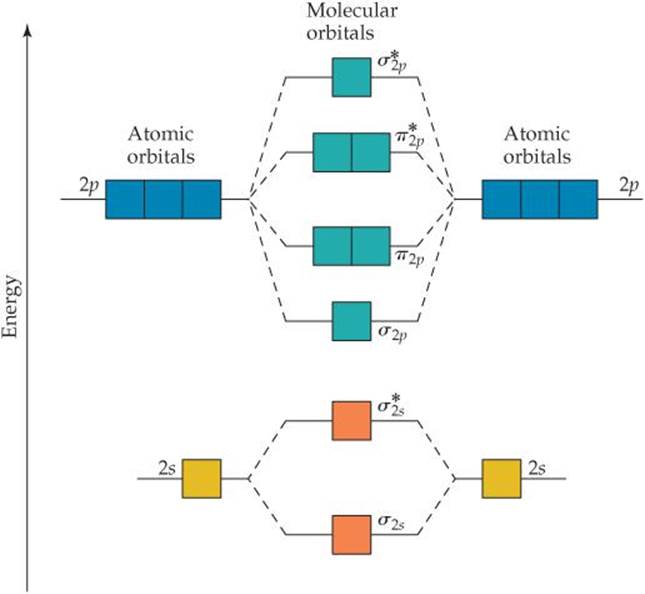
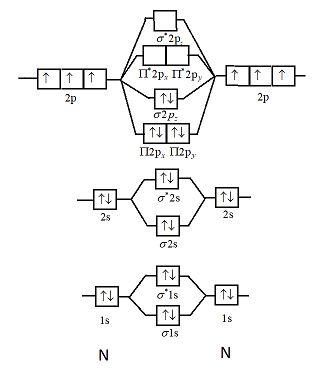
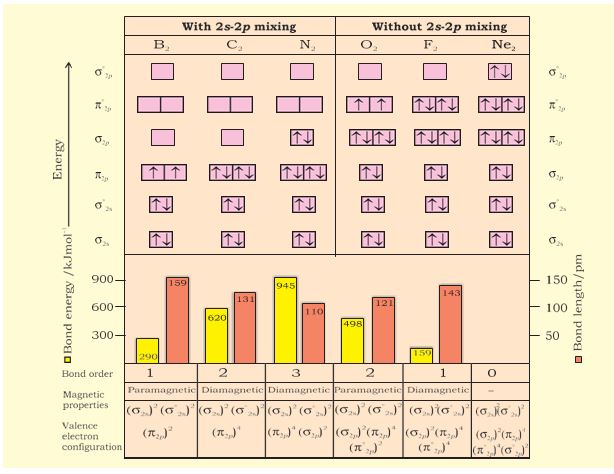
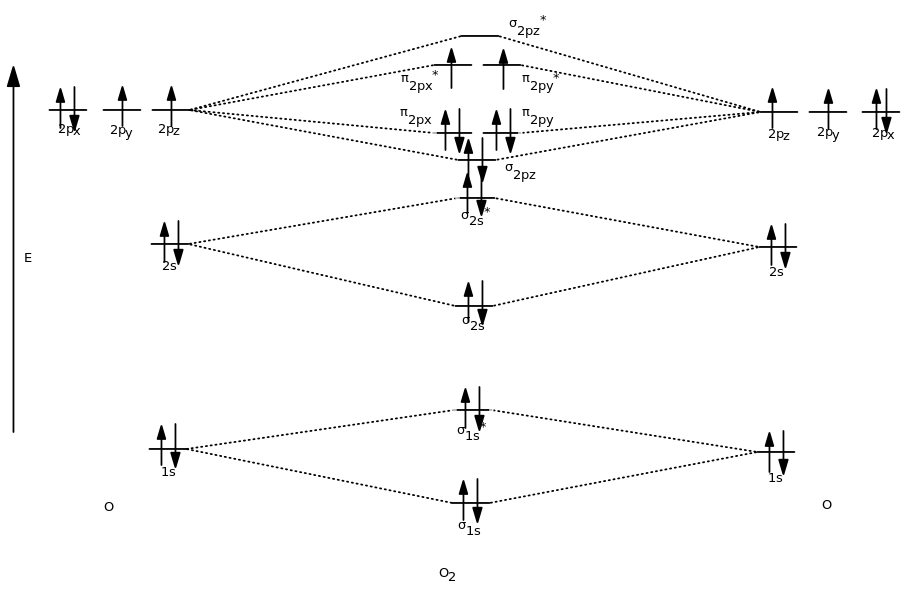
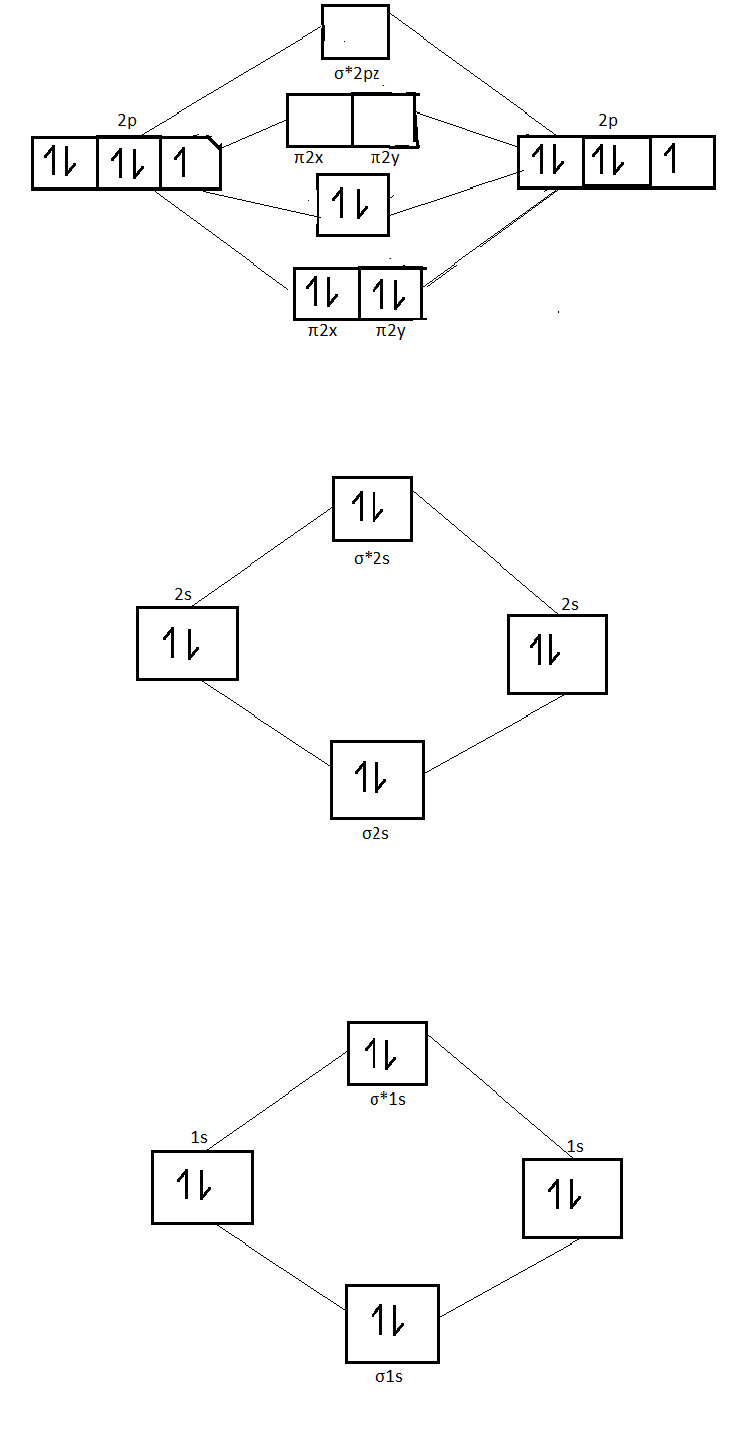
Ne2+ molecular orbital diagram. Molecular orbital theory predicts the stability of covalent bonds from the bond order of the molecule, which is the number of electrons in bonding orbitals minus the number of electrons in antibonding orbitals divided by two. A bond order of greater than zero indicates that one or more covalent bonds can exist, whereas a bond order of zero ... Molecular orbital confuguration of Ne2 is σ1s²σ*1s²σ2s²σ*2s²σ2Pz²π2Px²π2Py²π*2Px²π*2Py²σ*2Pz² Hence the bond order of Ne2 according to M.O.T is = (Nb-Na)/2 = (10-10)/2 (since,both bonding orbitals and non-bonding orbital contains 10 electrons) =0 Hence, no bond is possible between 2 Ne atom. Therefore, formation of this molecule is not possible. B) Ne2^2+ C) O2^2+ D) F2^2+ E) None of the above are paramagnetic; 3) Draw the molecular orbital diagram needed, and determine which of the following is paramagnetic. A) B2^2+ B) B2^2-C) N2^2+ D) C2^2-E) B2; 4) Draw the molecular orbital diagram shown to determine which of the following is most stable. Molecular orbital diagram ne2. If ne 2 did form, it would be diamagnetic. If ne 2 did form, it would be diamagnetic. Once you have the molecular orbitals and their energy ordering the ground state configuration is found by applying the pauli principle, the what is the molecular orbital diagram for the diatomic neon molecule, ne2?
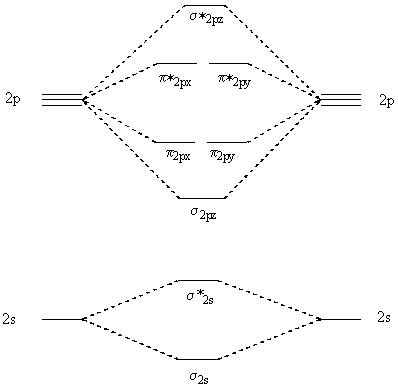
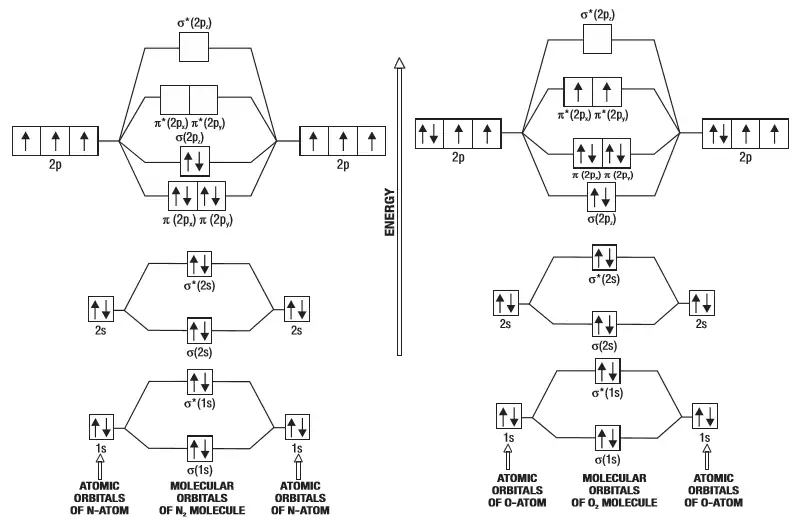
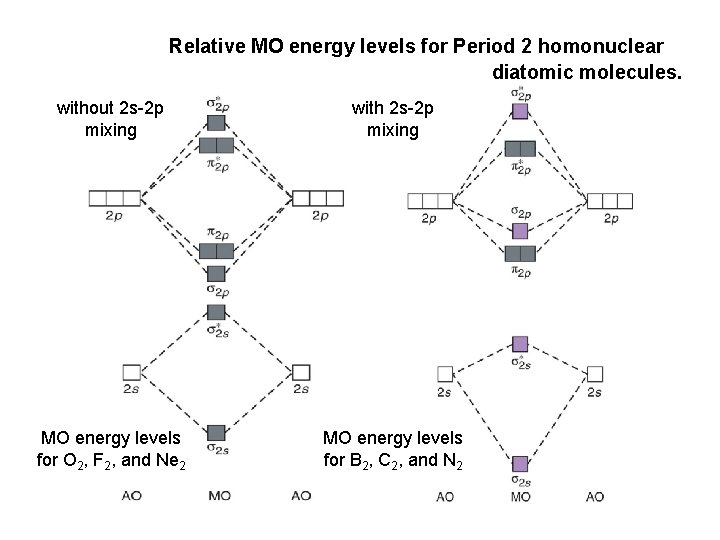
April 29, 2020 - Answer (1 of 4): Just from the position in the periodic chart, you should expect (circa chemistry from ~1900) to be non-reactive, including with itself. In terms of Lewis electron-dot structures (circa ~1920) you should expect neon to be non-reactive. From valence-bond theory (circa ~1935) you sh... November 21, 2018 - One is for the elements up to nitrogen. Ignoring weak clusters held together by van der waals interactions the reason that ne2 does not for... Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ... Chemistry questions and answers. Use the molecular orbital diagram shown below to determine which of the following molecules/species is most stable (O2, F2 and Ne2) . Explain their magnetic properties using the same diagram. Calculate the bond order in each of the molecule. Question: Use the molecular orbital diagram shown below to determine ...
5.7 a. The energy level diagram for NO is on the right. The odd electron is in a π2p* orbital. b ... Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in Free essays, homework help, flashcards, research papers, book reports, term papers, history, science, politics Mar 17, 2019 · Even rather simple molecular orbital (MO) theory can be used to predict from the bottom of the diagram because this is how MO diagrams are constructed, from N2, O2, F2, Ne2 the complexity of the molecular orbitals develop in two ways.Draw the molecular orbital diagram for Ne 2 + and determine if the bond between the two atoms will be stable.
0:21 Molecular Orbital Diagram of Oxygen Molecule3:30 Molecular Orbital Diagram of Florine Molecule5:25 Molecular Orbital Diagram of Neon MoleculeSo as we d...
Click here👆to get an answer to your question ✍️ Use the molecular orbital energy level diagram to show that N2 would be expected to have a triple bond, F2 , a single bond and Ne2 , no bond.
Answer (1 of 2): First let me make it clear that Ne, which is Neon, is a noble gas. Noble gases rarely form compounds and they don't exist as molecules in their pure form. So, Neon, as a gas, exists as only Ne and not as Ne2 (Oxygen, Hydrogen, Nitrogen are some of the many gases that exist as mol...
Molecular orbital energy level of Li2. MO diagrams for Diatomic Molcules. Overview. In this section, we will compare MO diagrams for diatomic molecules X-X, from Li2 to Ne2. Molecular orbital diagram of Li2 & Be2: Number of electrons in Li2 molecule =6. Li2 = σ1s2,σ*1s2,σ2s2. Nb=4, Na=2. B.O = (Nb- Na). B.O = ().
May 24, 2020 - Answer (1 of 4): Neon is denoted by Ne which is noble gas. Noble gases are non-reactive by nature.The outermost shell in Ne element is fully filled with electrons where the electron is 10 in number. This means that will have 20 electrons. This is why it does not need to react with any other atom ...
There are two MO diagrams you need to memorize for diatoms (N2, O2, Ne2, etc).One is for the elements up to Nitrogen. The other is for AFTER nitrogen (start...
First Year Chemistry in the School of Chemistry at the University of Sydney
From the periodic table as we have already discussed the Molecular orbital diagrams of diatomic molecules of 1st two periods starting from Hydrogen to Neon. ...
April 11, 2020 - There are two mo diagrams you need to memorize for diatoms n2 o2 ne2 etc. However this species has four valence electrons and its co...
According to Molecular Orbital theory, only those molecule can exists which have net positive bond order while the molecules with negative or. Answer to For Ne2, construct three molecular orbital diagrams, one each for the neutral molecule, the +1 cation, and the -1 anion. © Prof Adam J Bridgeman | close window.
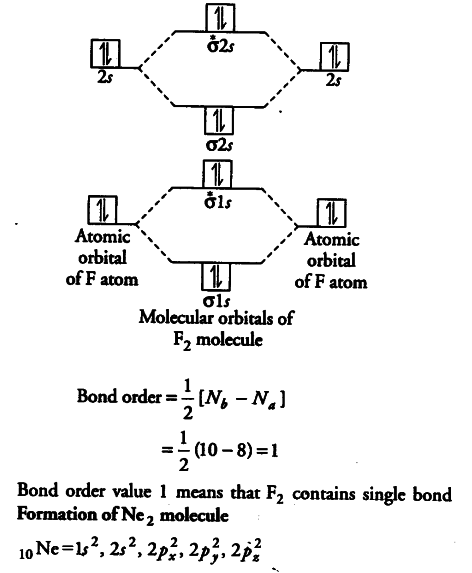
A molecular orbital explicitly describes the spatial distribution of a single electron orbitals, and σ∗. 1s is higher in energy. Draw this out using an energy level diagram: 2 He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. 2,. H−.A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical ...
Higher Education and Tertiary learning solutions that are specifically designed to accelerate learning, improve engagement and student outcomes
March 12, 2019 - A molecular orbital diagram or mo diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular ...
Molecular Orbital theory postulated by Robert.S. Mulliken illustrates the process of bonding held within the molecule. They can share two, four, or six electrons and form single, double, or triple bonds, respectively. Atoms form a bond by sharing electrons. Here in this article, we will discuss Molecular Orbitals, types of Molecular Orbitals, and their formation.
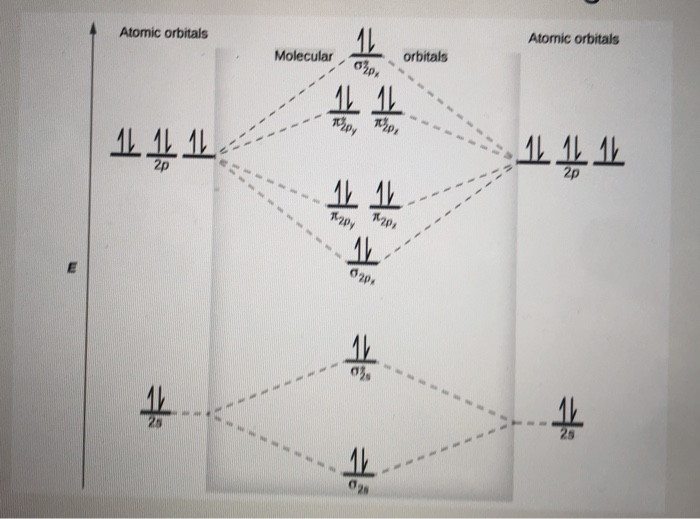
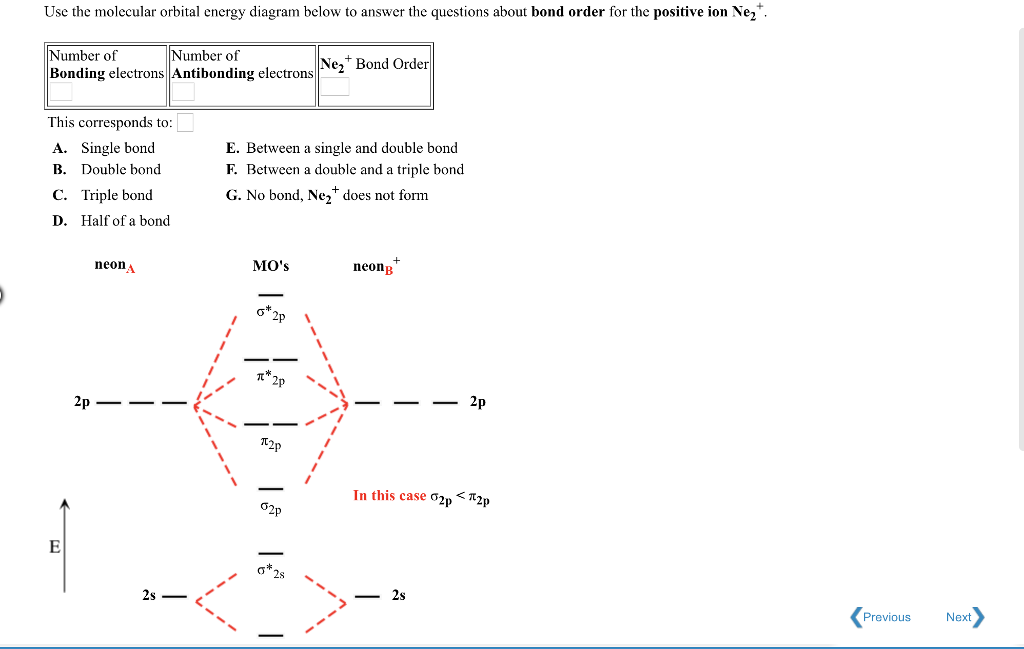
Question: O STRUCTURE AND BONDING Drawing the MO energy diagram for a Period 2 homodiat... Draw the molecular orbital (MO) electron diagram for the Ne2 molecule. Be sure your diagram contains all of the electrons in the molecule, including any core electrons. Energy
Remember: When two oxygen atoms bond, the pi(2p) bonding molecular orbitals are lower in energy than the sigma(2p) bonding orbitals. They are flipped compare...
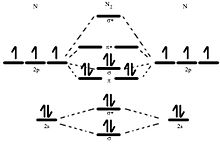
Molecular orbital diagram for nitrogen gas (N2)Use aufbau and Hund to fill with 10 valence electronsYou get sigma2s(2),sigma2s*(2),pi2p(4),sigma2p(2).Bond Or...
Answer to Draw the molecular orbital diagram for Ne2+ and determine if the bond between the two atoms will be stable. If 2p orbita...
Free essays, homework help, flashcards, research papers, book reports, term papers, history, science, politics
So here we're looking at the molecular orbital theory to describe bonding. So the first example, we have a C. Two plus, we've got 11 electrons and we can calculate the bond order which is the bonding electrons seven take away the anti bonding for divided by two.
Molecular Orbital Diagram for Nitrogen Gas (+1 ion) (N2(+)).Fill from the bottom up, with 9 valence electrons total.Bonding Order is 2.5, so it is unstable, ...
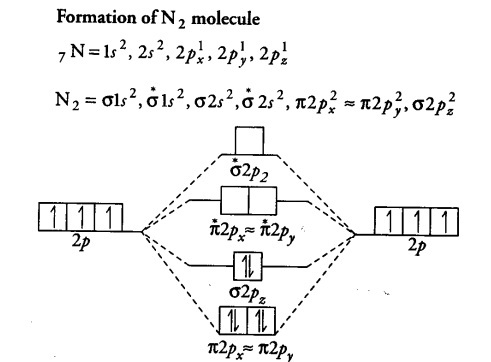
Formation of molecular orbitals can be determined by LCAO (linear. Continue Reading. Let me explain the molecular orbital diagram of N2 using its diagram. one atom of nitrogen has 7 electrons so a N2 molecule will have 14 electrons. so first 2 electrons go in 1s sigma bond. next 2 in 1s sigma anti bond orbital.
Molecular orbital diagram for ne2 . Bond order = [(number of bonding electon â number of antibonding electron)/2] Now, for N2â it is 2.5 See the MO diagram of N2â It is defined as the heat of formation for ions of opposite charge in the gas phase to combine into an ionic solid. The graphical representation presented in Fig.
26. jan. 2020 ... With the help of molecular orbital diagram show that Ne2 cannot exist as stable molecule. (Atomic number of Ne = 10). chemical bonding ...
Molecular Orbital Diagram of O 2 Chapter 9 Section 6 When filling the MO levels, you have to: Count the number of valence electrons, Start with the lower energy orbitals first, Follow Hund's rule, and Pt t th t Dr. A. Al-Saadi 19 Put not more than two electrons in one MO. Molecular Orbital Diagram of N 2 Chapter 9 Section 6
Below is an MO diagram of a π bond using two p orbitals. A nodal plane is a plane of zero electron density formed when orbitals of opposite phases overlap. It ...
When two carbons atoms bond, the pi(2p) bonding molecular orbitals are lower in energy than the sigma(2p) bonding orbitals.C2(2-) has a bond order of 3, so i...
Answer to For Ne2, construct three molecular orbital diagrams, one each for the neutral molecule, the +1 cation, and the -1 anion....
Transcribed image text: "Use the filled in molecular orbital diagram for a neutral Ne2 molecule shown below to ask the following questions: 111 111 Choom What is the bond order for the 1+ cation of the Ne2 molecule (Ne2+)? What is the bond order for the 2+ cation of the Ne2 molecule (Ne22+)? Atomic orbitals Atomic orbitals Molecular ( 14 能、othinal) orbitals (11: T TT 斗 业网止业%"
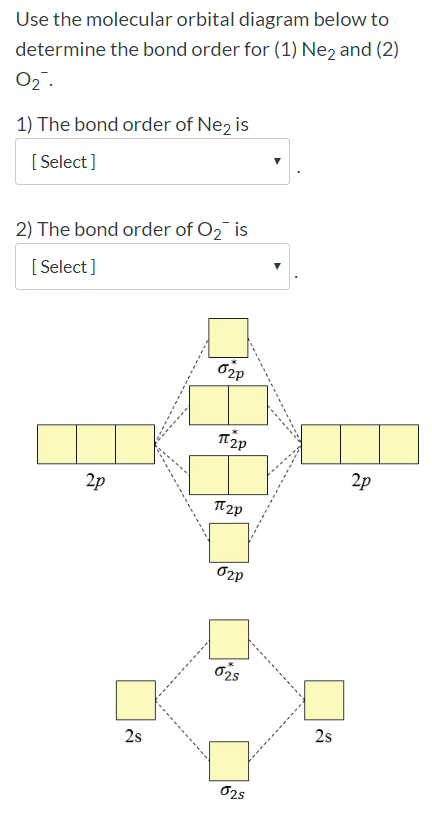
I only need the bond order for Ne2 Use the molecular orbital diagram below to determine the bond order for (1) Ne2 and (2) 022 1) The bond order of Nez is ...
the diagram above is the molecular.n2 molecular orbital energy level diagram picture, is usually depicted by a diatomic molecules chapter learn consider the molecular orbital electron configuration notation to a molecular orbitals diagrams web the molecular orbital energy level structures can construct the molecular orbital energy level the …
Molecular Orbital Diagram Ne2 28.12.2018 28.12.2018 7 Comments on Molecular Orbital Diagram Ne2 Even rather simple molecular orbital (MO) theory can be used to predict from the bottom of the diagram because this is how MO diagrams are constructed, from N2, O2, F2, Ne2 the complexity of the molecular orbitals develop in two ways.
February 4, 2014 - with the help of molecular orbital theory show that ne2 cannot exist as stable species - Chemistry - TopperLearning.com | yu03c344
Answer to For Ne2, construct three molecular orbital diagrams, one each for the neutral molecule, the +1 cation, and the -1 anion. A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining . Draw the Lewis Structure of Ne2. 1. Draw the atomic and hybrid orbitals on on side of the page.
In order to continue enjoying our site, we ask that you confirm your identity as a human. Thank you very much for your cooperation
With the help of molecular orbital diagram show that Ne2 cannot exist as stable molecule. (Atomic number of Ne = 10). asked Jan 26, 2020 in Chemistry by SurajKumar ( 66.2k points)
March 4, 2018 - Brainly.in is a part of the largest social network for studying in a group. We provide the best tools for mutual help with school subjects. Join us!


















0 Response to "39 ne2+ molecular orbital diagram"
Post a Comment