39 molecular orbital diagram for he2
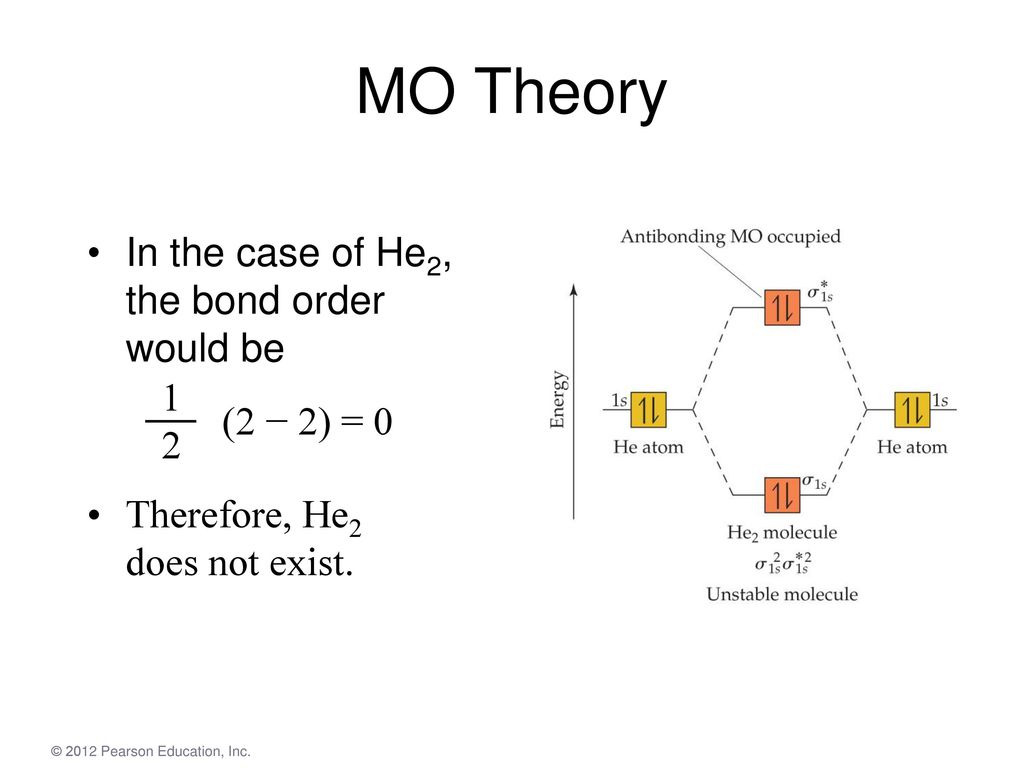
Solved Construct the molecular orbital diagram for He2 and Transcribed image text : Construct the molecular orbital diagram for He2 and then identify the bond order. Bond order: Click within the blue boxes to add electrons. 8.4 Molecular Orbital Theory - Chemistry Figure 10. The molecular orbital energy diagram predicts that He2 will not be a stable molecule, since it has equal numbers of bonding and Obtain the molecular orbital diagram for a homonuclear diatomic ion by adding or subtracting electrons from the diagram for the neutral molecule.
Introduction to Inorganic Chemistry/Molecular Orbital Theory... Valence bond (VB) theory gave us a qualitative picture of chemical bonding, which was useful for predicting the shapes of molecules, bond strengths, etc. It fails to describe some bonding situations accurately because it ignores the wave nature of the electrons.
Molecular orbital diagram for he2
Molecular Orbital Theory (MOT), Chemistry Study... | eMedicalPrep Introduction to Molecular Orbital Theory. Valence Bond Theory fails to answer certain questions like Why He2 molecule does not exist and why O2 is No. 9 Molecular Orbital Diagram for CO. Analysis done by Bond Order. If value of bond order is positive, it indicates a stable molecule and if the value... germanydating.expatica.comExpat Dating in Germany - chatting and dating - Front page DE Expatica is the international community’s online home away from home. A must-read for English-speaking expatriates and internationals across Europe, Expatica provides a tailored local news service and essential information on living, working, and moving to your country of choice. Molecular Orbitals: Molecular Orbital Theory | SparkNotes Figure %: An orbital correlation diagram for a hypothetical He-He molecule. From the orbital correlation diagram above you should notice that the Therefore, there is no net stabilization due to bonding so the He2 molecule will not exist. The bond order calculation shows that there will be a...
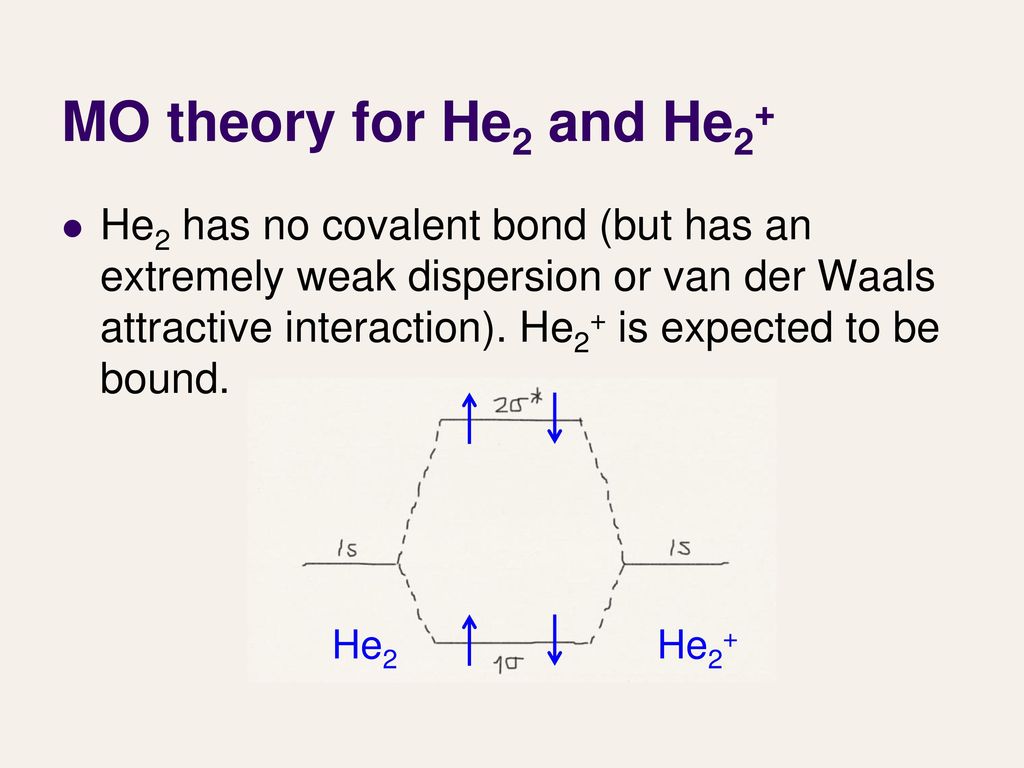
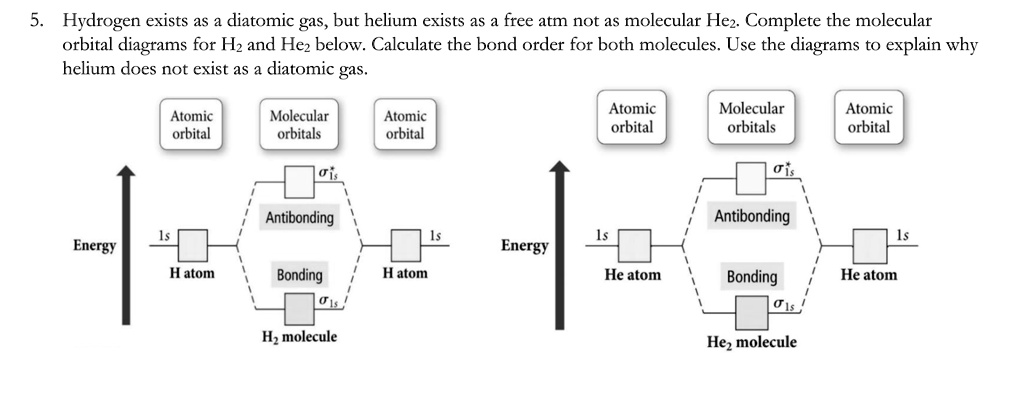
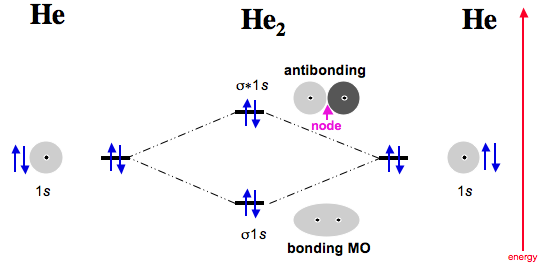
Molecular orbital diagram for he2. What is the molecular orbital diagram for B_2? | Socratic Before we can draw a molecular orbital diagram for B₂, we must find the in-phase and out-of-phase overlap combinations for boron's atomic orbitals. PDF Molecular Orbitals in | 9-2 Molecular Orbital Energy Level Diagrams In a bonding molecular orbital, the electron density is high between the two atoms, where it stabilizes the arrangement by exerting a strong attraction for both nuclei. The energy level diagram for He2 is similar to that for H2 except that it has two more electrons. He2 Molecular Orbital Diagram Molecular Orbital Diagram for Hydrogen Gas (H2). Fill from the bottom up, with 2 electrons total. Bonding Order is 1, and it is ... MOLECULAR ORBITAL THEORY Lecture 7 Containing below points:- 1. MO Energy Level Diagram for He2 molecule 2. MO ... Molecular Orbital Diagrams - Every Science We now consider a hypothetical molecule He2. This would also have two molecular orbitals formed from the overlap of 1s orbitals on the atoms, giving a molecular orbital diagram of the same appearance as the one above. However, since each He atom has two electrons in its outer shell...
MO Diagrams | Molecular Orbital Diagram Maker A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. The diagram is then completed by filling the energy levels with the correct number of electrons. The following molecules are currently available: Molecules of the First Row Draw and explain the molecular orbital diagram of... - Brainly.in Molecular orbital diagram of. Explanation: Neon atom has 10 electrons and its electronic configuration is . When molecule is considered, it has two neon atoms and thus is composed of 20 electrons. The electronic configuration and bond order of molecule is as follows › 35962833 › Clayden_Organic(PDF) Clayden Organic Chemistry (1) | angie ... - Academia.edu Academia.edu is a platform for academics to share research papers. CBSE Class 11 Chemistry Notes Download in PDF | Toppers ... CBSE Class 11 Chemistry Notes are Best ever notes prepared by our awesome team members. We have spend more that 2 years to prepare these Class 11 chemistry notes.After analyzing our notes in deep, we have uploaded our notes on the website.
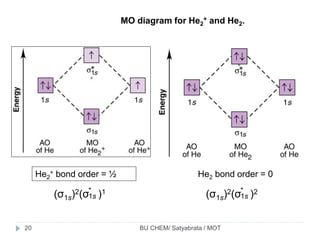
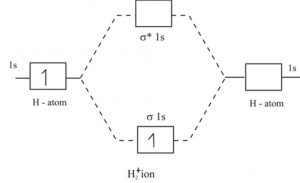
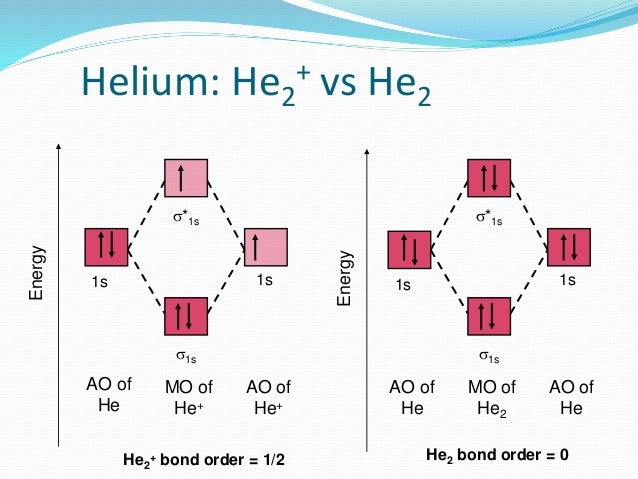
Molecular orbital diarams for He2 and He2+ - YouTube How to write simple Molecular Orbital Diagrams and determine the Bond order. Tutorial on Chemical Bonding, Part 8 of 10 (Molecular orbitals) Simple molecular orbital diagrams. Dihydrogen and its ion H2+. Dihelium He2. The molecular orbital model is by far the most productive of the various models of chemical bonding, and serves as the basis for most quantiative calculations, including those that lead to many of the... PDF Slide 1 Molecular orbital theory for diatomic molecules. Note for "He2" - extra electron in antibonding m.o. -therefore bond order = 0. Molecule does not exist - no force to hold atoms together. Molecular Orbital Energy Level Diagram for a Heteronuclear Diatomic. Why He2 is not in molecular form? - Quora According to the molecular orbital theory, in a supposed He2 molecule, both the bonding and the antibonding orbitals will have 2 electrons each. In order to predict the bond order, molecular orbital diagram for H2- is to be drawn. According to MOT number of atomic orbitals combined is equal to...
8.4 Molecular Orbital Theory - Chemistry 2e | OpenStax Figure 8.36 The molecular orbital energy diagram predicts that He2 will not be a stable molecule, since it has equal numbers of bonding and Obtain the molecular orbital diagram for a homonuclear diatomic ion by adding or subtracting electrons from the diagram for the neutral molecule.
Molecular Orbital Diagrams of Diatomic Molecules - Chem He2. Lewis Structure: Molecular Orbital Energy Diagram. Total # of bonding electrons. The orbital correlation diagram for diboron, however, is not generally applicable for all homonuclear diatomic molecules. It turns out that only when the bond lengths are relatively short (as in B2, C2, and N2) can...
Figure 14: The molecular orbital energy-level diagram for diatomic... Molecular orbital theory. Molecular orbitals of H2 and He2. The molecular orbital energy- level diagram that results is constructed by putting the molecular orbitals in order of increasing number of internuclear nodal planes, the orbital with no such nodal plane lying at lowest energy and the orbital...
Molecular orbital theory in He2, Li2 and F2 Flashcards | Quizlet Draw a molecular orbital diagram for He2, calculate the bond order and determine its stability. Show the shapes of the molecular orbitals also.
(PDF) Solid State ChemiStry and itS appliCation 2014 ... Solid State ChemiStry and itS appliCation 2014 Anthony R. West
dokumen.pub › general-chemistry-principles-andGeneral Chemistry: Principles and Modern Applications (10th ... What a Bonding Theory Should Do 450 Introduction to the Valence-Bond Method 451 Hybridization of Atomic Orbitals 453 Multiple Covalent Bonds 461 Molecular Orbital Theory 465 Delocalized Electrons: Bonding in the Benzene Molecule 474 Bonding in Metals 480 Some Unresolved Issues: Can Electron Charge-Density Plots Help? 484 Summary 489 Integrative ...
Molecular Orbital Theory | Boundless Chemistry The bonding diagram for the hypothetical molecule He2. Molecular orbital diagram for hydrogen: For a diatomic molecule, an MO diagram effectively shows the energetics of the bond between the two atoms, whose AO unbonded energies are shown on the sides.
› 34602222(PDF) Inorganic Chemistry Housecroft | Yurika ... - Academia.edu Pembuatan Senyawa kompleks asetial asetanoat. Enter the email address you signed up with and we'll email you a reset link.
Molecular Orbital Theory This molecular orbital model can be used to explain why He2 molecules don't exist. Combining a pair of helium atoms with 1s2 electron configurations would The molecular orbital diagram for an O2 molecule would therefore ignore the 1s electrons on both oxygen atoms and concentrate on the...
Molecular orbital diagram - WikiMili, The Best Wikipedia Reader A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital Dihelium (He-He) is a hypothetical molecule and MO theory helps to explain why dihelium does not exist in nature. The MO diagram for dihelium...
3 Ways to Calculate Bond Order in Chemistry - wikiHow 18.01.2022 · In molecular orbital theory, bond order is also defined as half of the difference between the number of bonding and antibonding electrons. For a straightforward answer: use this formula: Bond order = [(Number of electrons in bonding molecules) - (Number of electrons in antibonding molecules)]/2 .
Energy level diagram for Molecular orbitals - Chemical Bonding and... 3) If Nb = Na ,the molecule is again unstable because influence of electrons in the antibonding molecular orbital is greater than the bond influence of electron in the bonding molecular orbitals. No bond is formed between two helium therefore He2 does not exist.
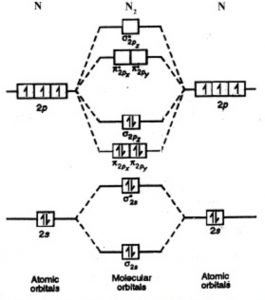
electronic configuration - Molecular orbital (MO) diagram for N2 and... I have been taught that the MO diagram is different for molecules with 14 or less electrons than the one used for molecules with 15 or more electrons. All this is simply because the primitive molecular orbital theory does not explain the things, rather it rationalizes them (for differences between...
Molecular Orbital Theory - ppt video online download 42 Molecular Orbital Diagrams Involving s and p Orbitals Identify the orbitals that represent the following Sigma bonds and antibonds Nonbonding molecular orbitals Antibonding molecular orbitals Pi bonds and antibonds Bonding molecular orbitals Weak pz-s mo interaction Homonuclear examples...
PDF Fig. 8-4. Molecular orbital energy level diagram for homonuclear... molecular orbital theory ascribes the instability of He2 to the equal occupation of bonding and antibonding orbitals. Notice that the Pauli exclusion principle is still the basic cause of the instability. If it were not for the Pauli principle, all four electrons could occupy a σg-type orbital and concentrate their...
Asked for: molecular orbital energy-level diagram, valence electron... s. Draw the molecular orbital energy-level diagram for the system. Determine the total number of valence electrons in the He22+ ion. A Because sodium has a [Ne]3s1 electron configuration, the molecular orbital energy-level diagram is qualitatively identical to the diagram for the interaction of...
PDF Microsoft Word - Handin8s2017ans.docx 1. Sketch the qualitative molecular orbital diagram for XeF2. The molecule is linear and symmetric. Assume the valence 5s-orbitals of Xe are sufficiently Determine the primary MOs that determine the bond order. Compare the general features of your MO diagram to the MO diagram for [F-H-F]...
Molecular orbital diagram - Wikipedia A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular.
(PDF) Vollhardt Organic Chemistry Structure Function 6th ... Vollhardt Organic Chemistry Structure Function 6th txtbk.PDF. Download. Vollhardt Organic Chemistry Structure Function 6th txtbk.PDF
PDF Microsoft PowerPoint - An introduction to Molecular Orbital Theory.ppt... - MO diagrams for Inorganic complexes. • It is a waste of both the lecturers and students time if the tutorial to ends up being a lecture covering questions. 5. An introduction to Molecular Orbital Theory.
Molecular Orbitals: Molecular Orbital Theory | SparkNotes Figure %: An orbital correlation diagram for a hypothetical He-He molecule. From the orbital correlation diagram above you should notice that the Therefore, there is no net stabilization due to bonding so the He2 molecule will not exist. The bond order calculation shows that there will be a...
germanydating.expatica.comExpat Dating in Germany - chatting and dating - Front page DE Expatica is the international community’s online home away from home. A must-read for English-speaking expatriates and internationals across Europe, Expatica provides a tailored local news service and essential information on living, working, and moving to your country of choice.
Molecular Orbital Theory (MOT), Chemistry Study... | eMedicalPrep Introduction to Molecular Orbital Theory. Valence Bond Theory fails to answer certain questions like Why He2 molecule does not exist and why O2 is No. 9 Molecular Orbital Diagram for CO. Analysis done by Bond Order. If value of bond order is positive, it indicates a stable molecule and if the value...



















0 Response to "39 molecular orbital diagram for he2"
Post a Comment