40 c2h4 molecular orbital diagram
Generate the Molecular Orbitals for CH4 (Td), CH4 (D4h) and Cyclopropane using diagram between the bonding MOs of square planar and tetrahedral CH4. The molecular orbital description of bonding in methane does several things for us. Here is an energy level diagram showing how the 4 hydrogen 1s orbitals.Jan 18, · Using LCAO to Construct MOs for ... 16. In valence bond theory, the pi bond between the two carbon atoms in C2H4 results from: (1) the overlap of the 2s orbitals from each carbon atom. (2) the overlap of a 2s orbital from one carbon atom with a 2p orbital from the other carbon atom (3) the above and below the molecular plane overlap of a 2p orbital from each carbon atom.
Polyatomic Molecular Orbital Theory Transformational properties of atomic orbitals Atomic orbital Transforms as s x2+y 2+z 2 px x py y pz z dz2 z2, 2z 2-x2-y2 dx2-y2 x2-y2 dxy xy dxz xz dyz yz S py • When bonds are formed, atomic orbitals combine according to their symmetry. • Symmetry properties and degeneracy of orbitals and bonds can be ...
C2h4 molecular orbital diagram
• Name the molecular geometry from the atom positions ... overlap of two p-orbitals pointing in the same direction. So, a double bond contains 1σ + 1π bond and a triple bond contains 1σ + 2π bonds. e.g. CH 4 and C2H6 contain all σ-bonds. Ethylene, C2H4 has the Lewis Structure: Derivation of the sigma molecular orbitals of XeF4 by the projection operator method.0:15 Structure of xenon tetrafluoride1:38 Projection operator table5... C2h4 Molecular Orbital Diagram 06.10.201806.10.20183 Commentson C2h4 Molecular Orbital Diagram a complex MO diagram: B2H6 MO diagrams combine two fragments. Symmetry fragments . these are MOs from C2H4 which belongs to the D2h point group. Ethene C2H4 molecular orbitals.
C2h4 molecular orbital diagram. Molecular orbital (MO) theory was developed by F. Hund and R.S. Mulliken in 1932. The salient features of this theory are: The electrons in a molecule are present in the various molecular orbitals as the electrons of atoms are present in the various atomic orbitals. The atomic orbitals of camparable energies and proper symmetry combine to form ... Molecular formula = C2H4 Empirical formula = CH2 Molecular mass = 28 ... Due to Sp2-hybridization each C-atom generates three Sp2-hybrid orbitals. In this way there exist six Sp2-hybrid orbital. These ... C2H5OH C2H4 + H2O OR Ethyl alcohol may ... However, carbon will be the central atom and its orbitals will take part in hybridization. During the formation of C2H6, 1 s orbital and px, py, and pz orbitals undergo sp 3 hybridization. This results in the formation of four hybridized orbitals for each carbon atom. The molecular hybrid orbitals now form different bonds between the electrons. Instead of single symmetry group approach treated in literature, method of cascade symmetry is applied to molecular orbitals of C2H4. Cascade symmetry method means that C2v group symmetry of lower ...
The resulting molecular shape is bent with an H-O-H angle of 104.5°. How many hybrid orbitals are in C2H4? Hybridization of Ethene ( C2H4 ) C2H4 has an sp2 Hybridization process. In this Hybridization one 's' and two 'p' orbitals are mixed to give three new sp2 hybrid orbitals which all are in the same shape and equivalent energies. The molecular orbital wave function for the electrons is constructed from a linear combination of (normalised) p orbitals from the atoms. In Carbon, each atom contributes one such electron. All overlap integrals are neglected. The (diagonal) energy integral, , is the same for all atoms i (of the same element). C2H4. π Molecular Orbitals of Ethene. is the coefficient in the molecular orbital of the atomic wavefunction from the i-th atom. We draw a molecular orbital energy diagram similar to that shown in Figure 11. Ethene from above the trigonal plane. Being A True Filipino Essay ... 2 Lecture 2 Pi bond (π): bonding molecular orbital -The bonding electron density lies above and below, or in front and in back of the bonding axis, with no electron directly on the bonding axis, since 2p orbitals do not have any electron density at the nucleus.
The above-mentioned molecular orbital diagram of acetylene (C2H2) is specifically showing the Carbon-Carbon bond. You can see how the sp hybridized orbitals combine and overlap to form a bonding sigma (σ) orbital and an antibonding sigma (σ*) orbital. Molecular Orbital (MO) Theory (continued 1) • Filling of MOs with electrons is governed by the same rules as for atomic orbitals • Aufbau principle - Fill MOs beginning with the lowest energy unoccupied molecular orbital • Pauli exclusion principle - No more than two electrons can be accommodated in a MO, and their spins must be paired C2H4 Lewis Structure, Molecular Structure, Hybridization, Bond Angle and Shape. The chemical formula C2H4 represents Ethylene. This molecule is also represented by H2C=CH2, clearly showing the alkene nature of the compound. An alkene is a hydrocarbon with a Carbon-Carbon double bond. C2H4 exists as a colorless gas and is flammable. The molecular structure has been optimized at the B3LYP/6-31g* level of theory. Charges used for electrostatic maps are computed using the NBO method. The molecular vibrations are
Mathematically, the formation of molecular orbitals may be described by the linear combination of atomic orbitals that can take place by addition and by subtraction of wave functions of individual atomic orbitals as shown below: Therefore, the two molecular orbitals o and o*are formed as : ... C2H4 Hybridization. July 30, 2019
construct a molecular orbital diagram of C2H4 with molecular obital digrams of CH2 on both sides of C2H4. I think I have CH2 correct but I am not too sure how to combine a CH2 and CH2 to make C2H4 in the middle. Please post detailed explaination. I have included my CH2 picture. Show transcribed image text.
Ethene C2H4 molecular orbitals. Loop Diagram. From this diagram, calculate the bond order for O 2. Many molecular orbital diagrams are not made up from atomic orbitals, but from fragment molecule (C2H4) is like the dxz AO and hence has b2g symmetry.
In ethylene, each carbon combines with three other atoms rather than four. There is a formation of a sigma bond and a pi bond between two carbon atoms. C2H4 Molecular Geometry And Bond Angles C2H4 molecular geometry is said to be planar in structure while the sp 2 orbitals are placed at a bond angle of 120 o.
Answer to construct a molecular orbital diagram of C2H4 with molecular obital digrams of CH2 on both sides of C2H4 I think I have. Loop Diagram. 3dxy . Construct the molecular orbital diagram for dichlorine. C2H4. Ethene from above the trigonal plane. The carbon atoms and orbitals are. Ethylene is the simplest molecule that has a double bond.
Ethene, which is two carbon atoms double bonded and two hydrogen atoms on EACH carbon (four hydrogen atoms total), requires ONE pi bond. This means each carb...
C2H4 Molecular Orbital (MO) Diagram The molecular orbital theory is a concept of quantum mechanics where atomic linearly combines to form molecular orbitals and we describe the wave nature of atomic particles. Here, bond strength depends on the overlapping degree which in turn depends on the spatial proximity of the combining atoms.
HYBRIDIZATION THEORY, & MOLECULAR ORBITALS ORBITAL COMBINATIONS Atomic orbitals can be combined and reshaped -much like dough- to make other orbitals of different shapes and properties. There are two basic types of orbitals that can result from such processes. They are: 1. HYBRID ORBITALS.
An antibonding molecular orbital is higher in energy than the atomic orbitals of which it is composed. 1 and 3 The C—C—H bond angles in ethylene, C2H4, are 120°.
C2h4 Molecular Orbital Diagram 06.10.201806.10.20183 Commentson C2h4 Molecular Orbital Diagram a complex MO diagram: B2H6 MO diagrams combine two fragments. Symmetry fragments . these are MOs from C2H4 which belongs to the D2h point group. Ethene C2H4 molecular orbitals.
Derivation of the sigma molecular orbitals of XeF4 by the projection operator method.0:15 Structure of xenon tetrafluoride1:38 Projection operator table5...
• Name the molecular geometry from the atom positions ... overlap of two p-orbitals pointing in the same direction. So, a double bond contains 1σ + 1π bond and a triple bond contains 1σ + 2π bonds. e.g. CH 4 and C2H6 contain all σ-bonds. Ethylene, C2H4 has the Lewis Structure:














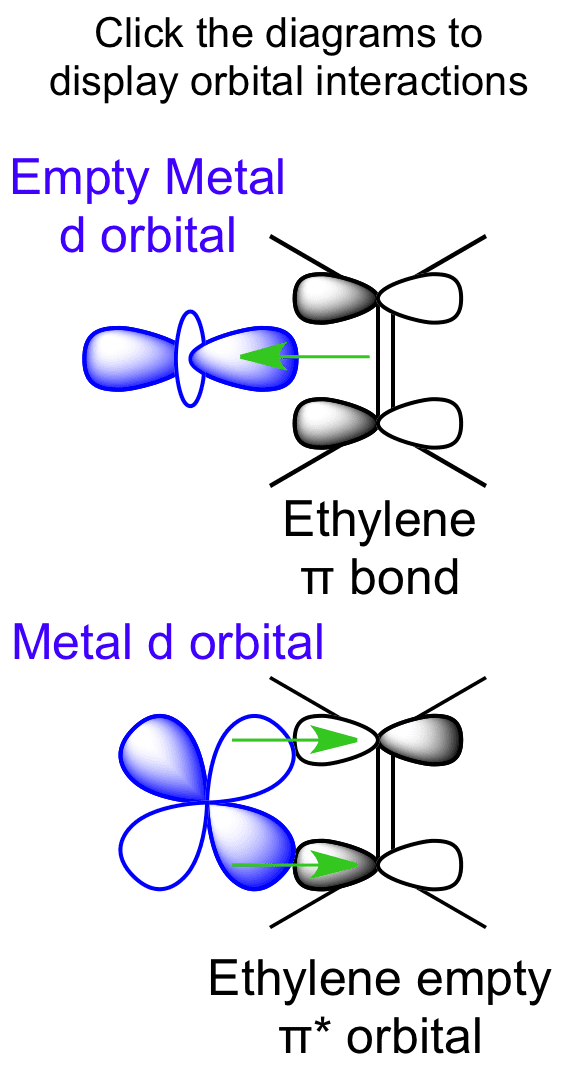

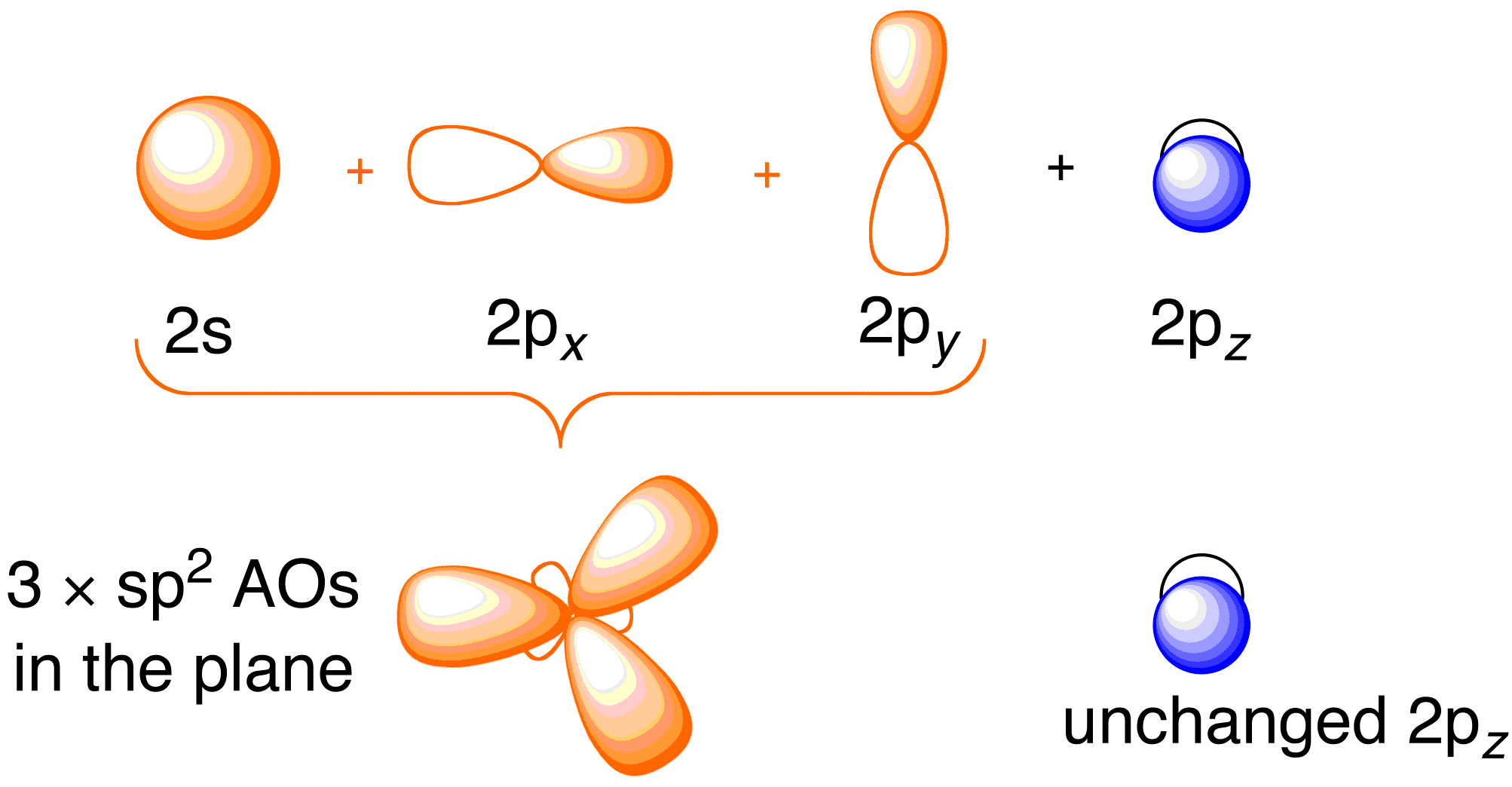




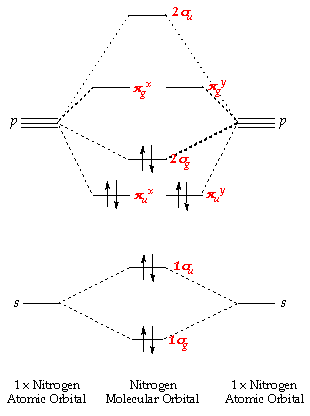
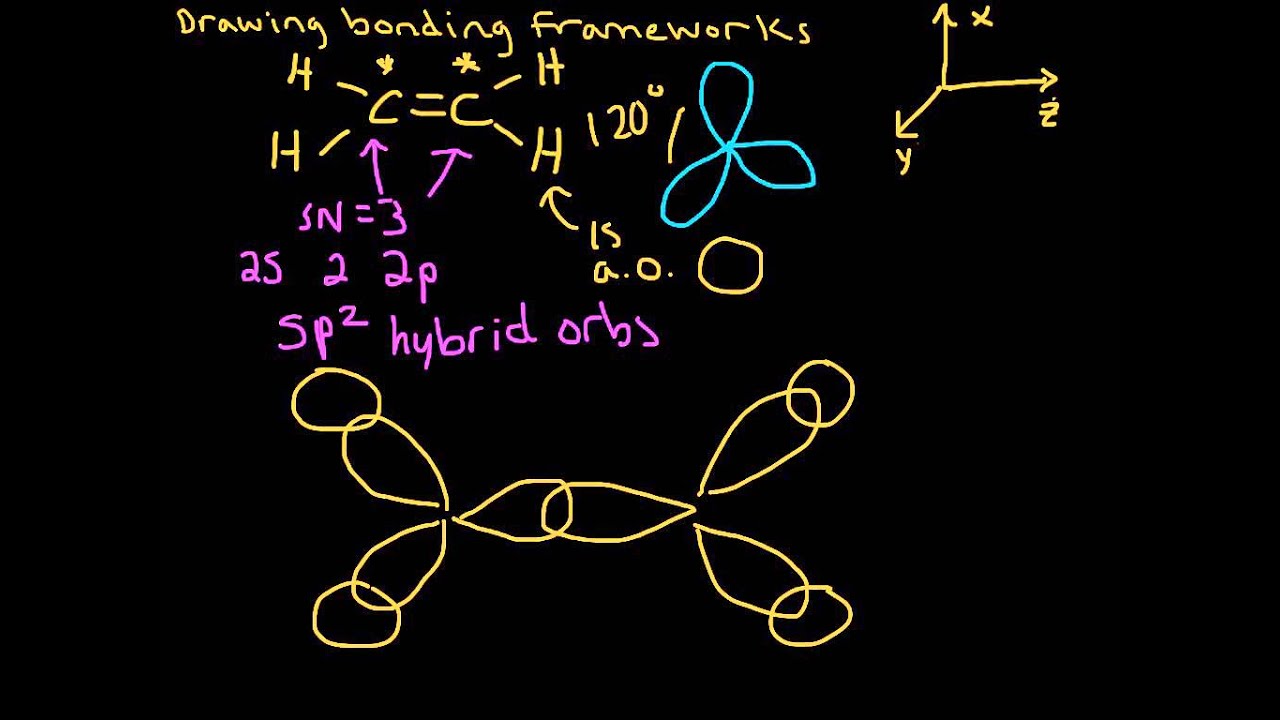


0 Response to "40 c2h4 molecular orbital diagram"
Post a Comment