40 gibbs free energy diagram
Understanding Gibbs Free Energy - Surfguppy - Chemistry made easy... Gibbs free energy is used to predict whether a chemical process is spontaneous or non-spontaneous. You might be wondering why we need to know that. Well, there are many applications where advance knowledge of whether a chemical reaction will proceed or not is important. Gibbs Free Energy | Boundless Chemistry Standard Gibbs free energies of formation are normally found directly from tables. Once the values for all the reactants and products are known, the standard Gibbs free energy change for the reaction can be found. Most tables of thermodynamic values list ΔGf°'s for common substances.
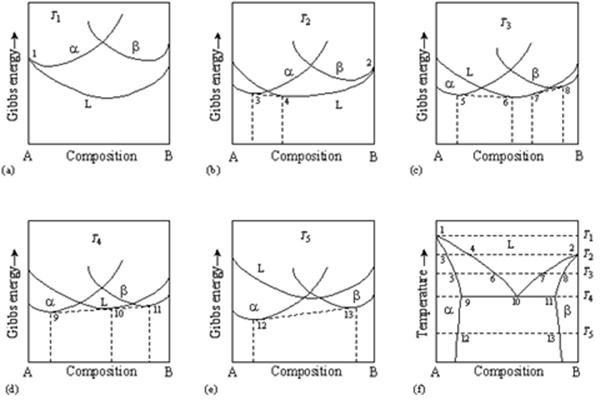
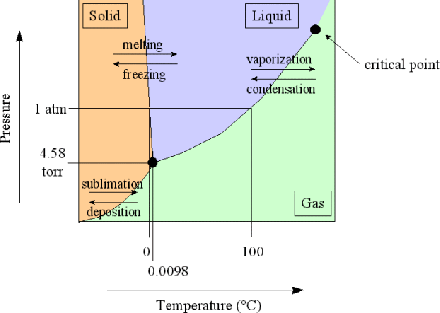
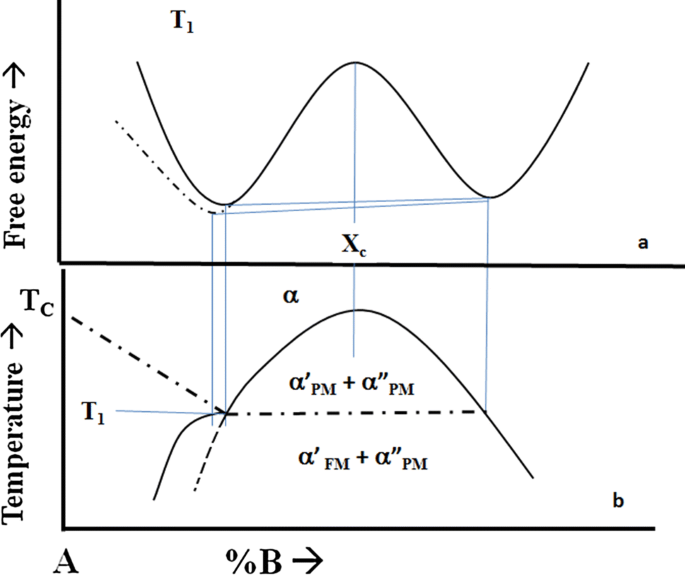
PDF 10. 6 Phase Diagrams, Gibbs Free Energy, and Thermodynamic... Gibbs Free Energy Composition and Phase Diagrams of Binary Systems. Figure 10.20 The Gibbs free energy of mixing curves at various temperatures, and the phase diagram for a binary system which forms regular solid solutions in Which Ω=0 and regular liquid solutions in which Ω...
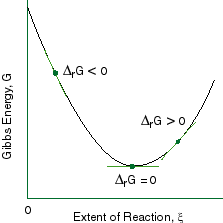
Gibbs free energy diagram
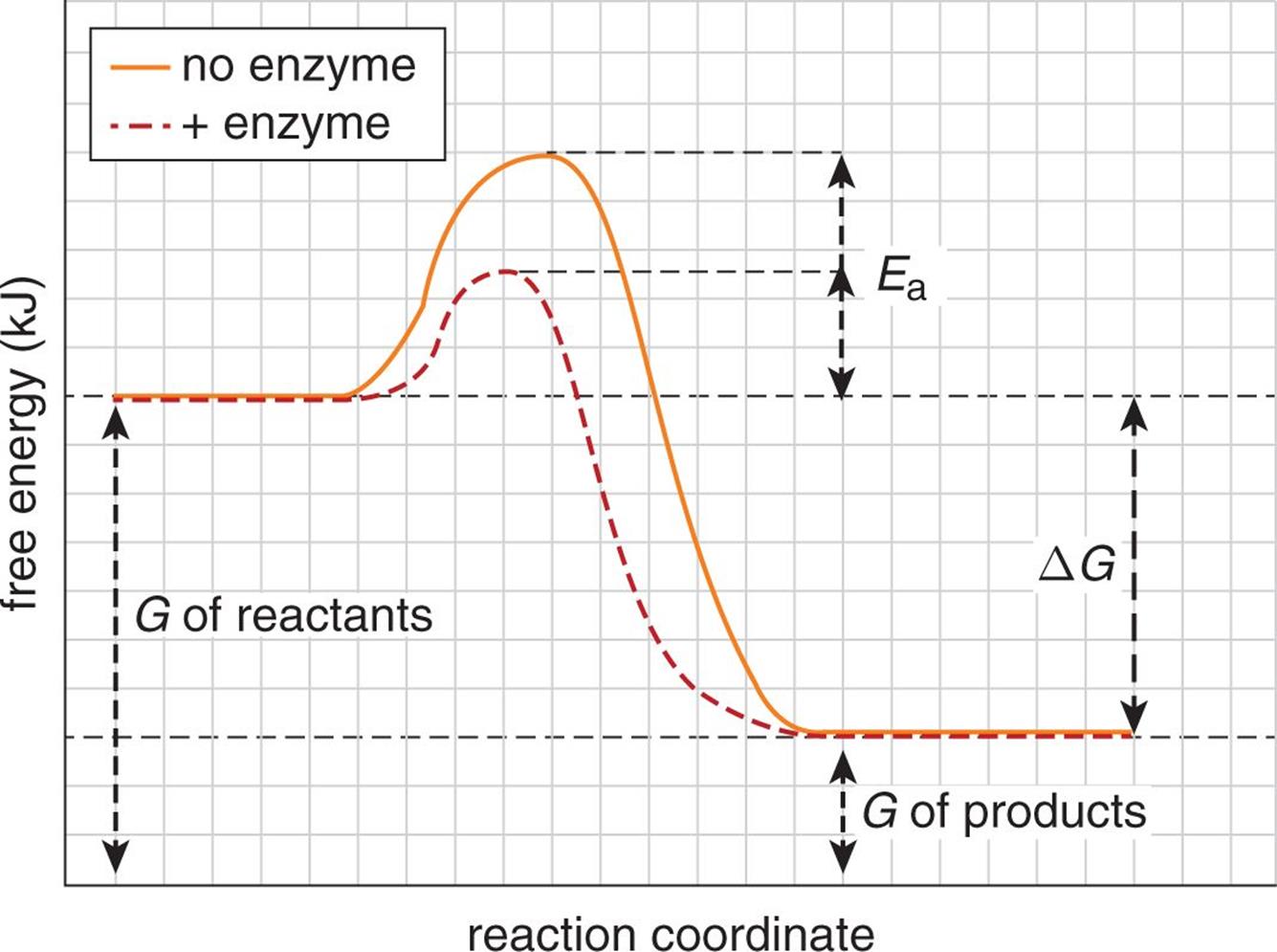
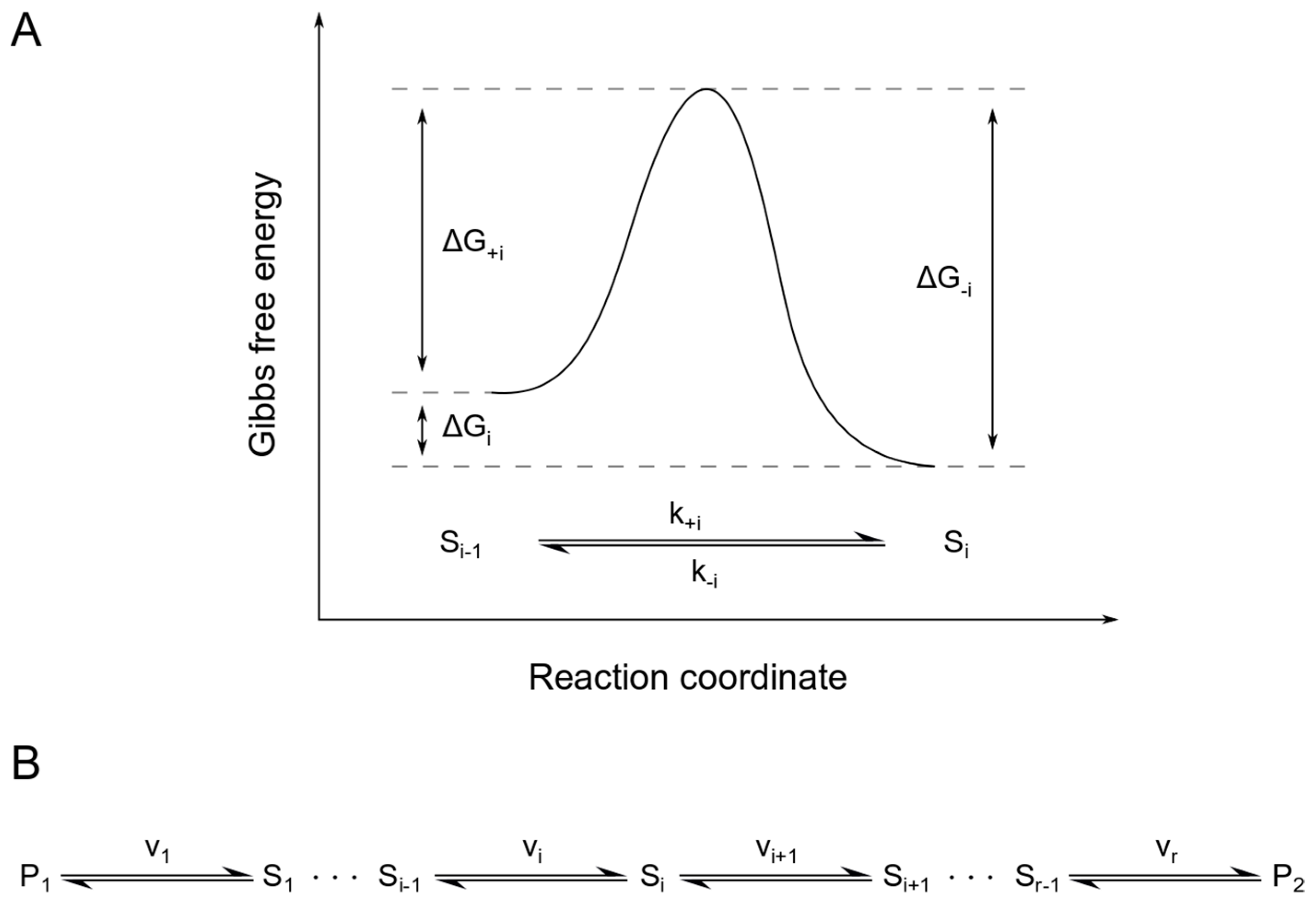
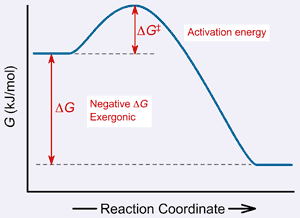
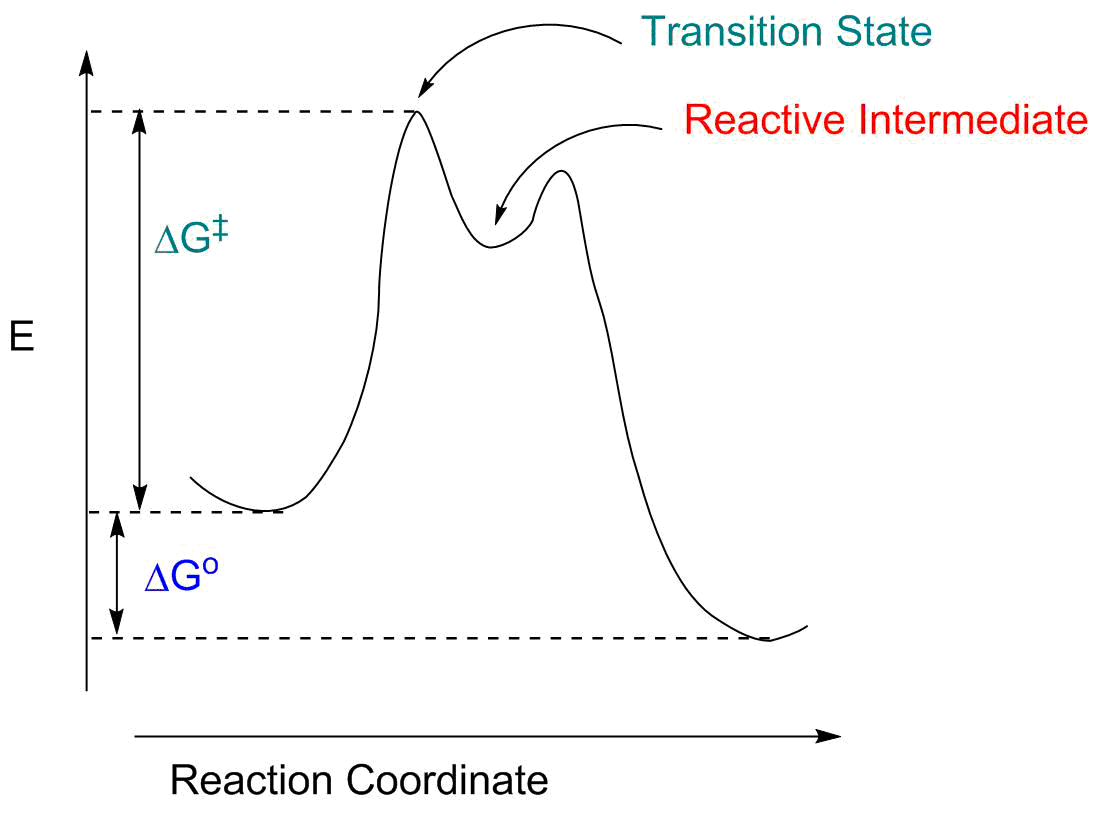
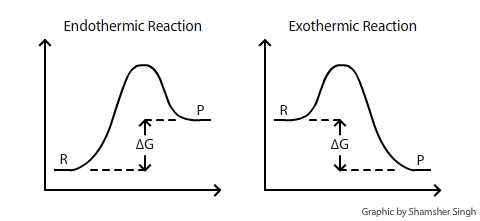
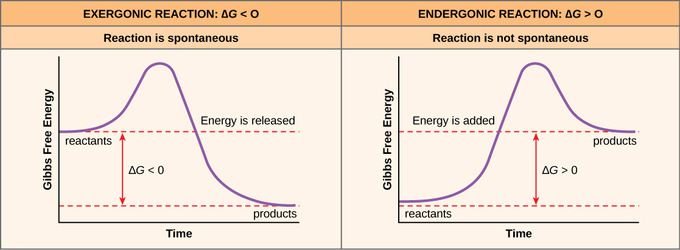
Structural Biochemistry/Enzyme/Gibbs free energy graph - Wikibooks... The Gibbs free energy graph shows whether or not a reaction is spontaneous-- whether it is exergonic or endergonic. ΔG is the change in free energy. Generally, all reactions want to go to a lower energy state, thus a negative change is favored. Gibbs Free Energy Formula : Structure, Preparations and Properties Gibbs Free Energy Formula- In thermodynamics, the Gibbs free energy or IUPAC name Gibbs energy or Gibbs function or free enthalpy is the thermodynamic potential. We use it to calculate the maximum reversible work that a thermodynamic system performs at constant pressure and... Gibbs Free Energy Gibbs Free Energy is used to determine whether a reaction is favored or disfavored. It is given by the equation Even if a reaction has a positive ΔG, it can be driven by an external source of energy, such as an electric current, photons of light, or by coupling to a spontaneous reaction.
Gibbs free energy diagram. Gibbs Free Energy curves and Phase diagrams for... - BragitOff.com Eutectic Phase diagram and Gibbs free energy curves. Gibbs Free Energy Free Energy and Free Energy Change—the Gibbs free energy, G, is used to describe the spontaneity of a process. The free energy change, DG is equal to -TDSuniv and it applies just to a system itself, without regard for the surroundings. It is defined by the Gibbs equation Gibbs free energy - Wikiwand In thermodynamics, the Gibbs free energy (or Gibbs energy) is a thermodynamic potential that can be used to calculate the maximum reversible work that may be performed by a thermodynamic system at a constant temperature and pressure. Gibbs Free Energy and Energy Diagrams Explained - GAMSAT... Hey everyone, in this video I'll be covering what Gibbs free energy is, how it's calculated and where it fits in on an energy diagram. I'll also be...
An introduction to Gibbs free energy and its use in predicting the... This page introduces Gibbs free energy (often just called free energy), and shows how it can be used to predict the feasibility of reactions. If you have already read the page about how to do this with total entropy changes, you will find a little bit of repetition on this page. Gibbs_free_energy Gibbs free energy Thermodynamic potentials Internal energy U(S,V) Helmholtz free energy A(T,V) Thus, Gibbs free energy is most useful for thermochemical processes at constant temperature and Such processes don't move on a P-T diagram, such as phase change of a pure substance, which... Gibbs Free Energy | About the above diagrams 1 Free energy: the Gibbs function. 2 Free energy and chemical change. Physical meaning of free energy. The important role of temperature. The maximum work. 3 The standard Gibbs free energy. What do we mean by "standard"? Defining ΔG° and ΔGf°. Gibbs free energy | Tree of Knowledge Wiki | Fandom In thermodynamics, the Gibbs free energy (IUPAC recommended name: Gibbs energy or Gibbs function; also known as free enthalpy to distinguish it from Helmholtz free energy) is a thermodynamic potential that can be used to calculate the maximum of reversible work that may be performed by a...
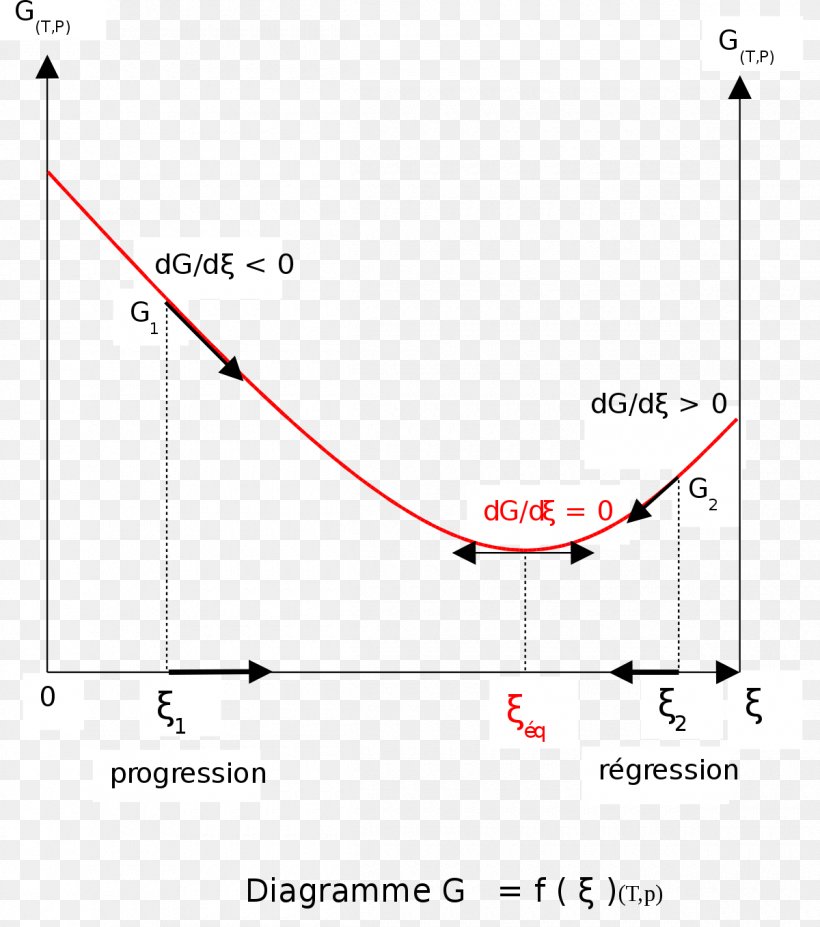
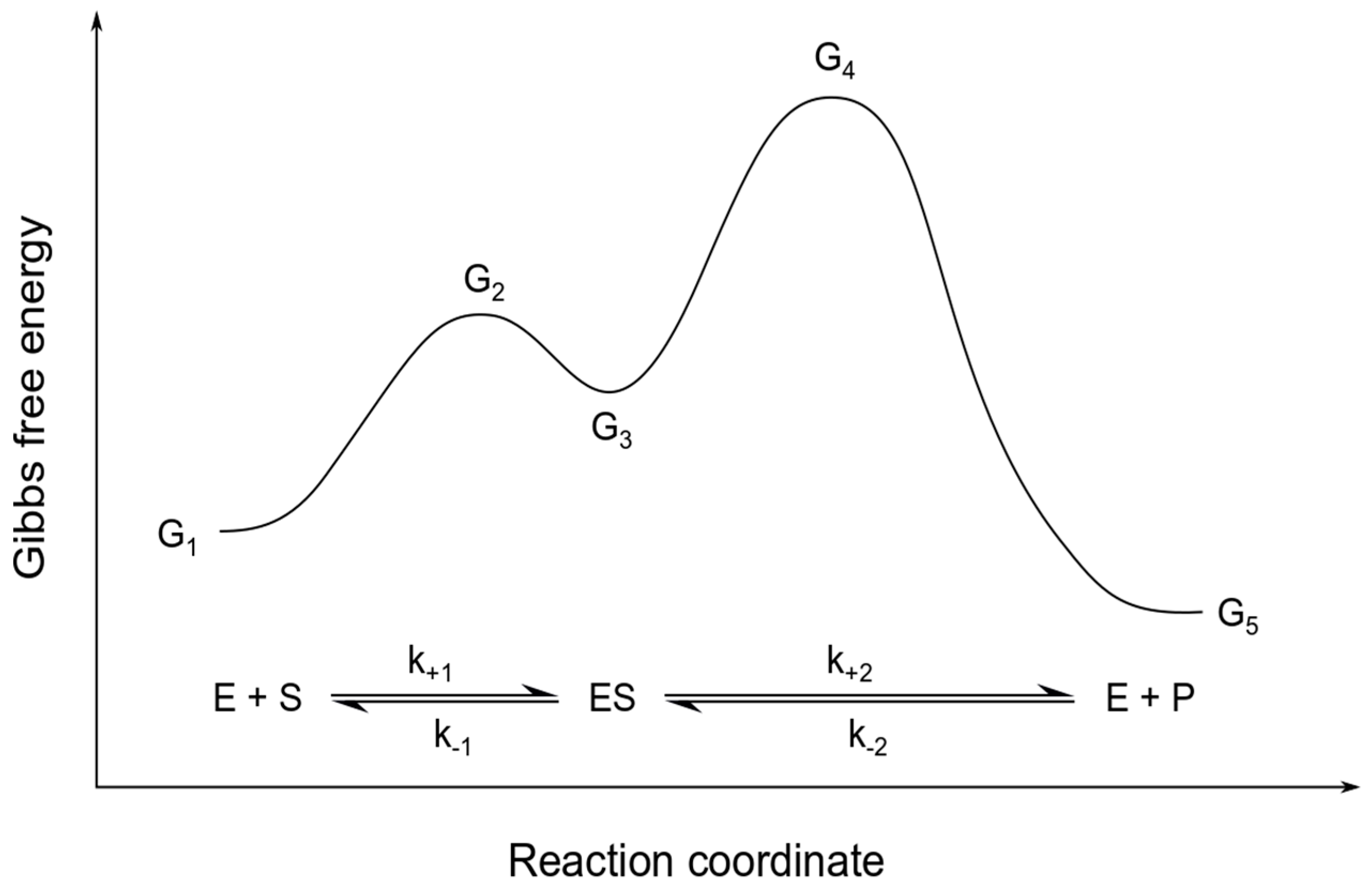
Gibbs Free Energy Gibbs Free Energy. The American physicist Josiah Gibbs introduced (ca. Although the free energy change of a reaction can be calculated from the preceding equation, if Δ H and Δ S for the reaction are not known, it is much more common to use this alternative equation Gibbs Free Energy The Gibbs free energy of the system is a state function because it is defined in terms of thermodynamic properties that are state functions. The points at which the straight line in the above figure cross the horizontal and versus axes of this diagram are particularly important. Gibbs Energy Gibbs free energies are relative values, not absolute values. They allow us to compare energies of different phases but individual values by themselves have no significance. The Gibbs Free Energy of any phase varies with pressure and temperature. The fundamental relationship is Gibbs Free Energy - an overview | ScienceDirect Topics Gibbs' free energy of mixing is released by the system when two different salinity solutions mix together. Gibbs' free energy of mixing can be used to explain the near-isobaric behavior of the PRO processes, where the released energy compensates for the pressure drop, which is a result of the...
PDF Equilibrium Phase Diagrams | Gibbs free energy Gibbs free energy. X First law of thermodynamics: conservation of the energy in any process. Introduction of the internal energy E Introduction of the From the Gibbs free energy variations of the phases, G(X,T), to the determination of the phase diagram (X: Composition, T: Temperature).
Gibbs Free Energy ΔG= Gibbs Free Energy ΔH = Enthalpy T = temperature in Kelvin ΔS = ENTROPY. Physics Definition-Gibbs free energy, the amount of thermodynamic energy in a fluid system which can be converted into non-mechanical work at a constant temperature and pressure.
Gibbs Free Energy - Chemistry, Class 11, Thermodynamics Gibbs free energy is that thermodynamic quantity of a system the decrease in whose value during a process is equal to the maximum possible useful work that can be obtained from the system. The relationship between heat absorbed by a system q, the change in its internal energy , ΔU...
Gibbs free energy changes equation calculations reaction feasibility... Part 3.4 Introduces the Gibbs free energy equation for determining the feasibility of a reaction. The ideas and equation are applied to any chemical changes and more specifically the extraction of metals (e.g. feasibility of carbon reduction of metal oxide) and the direction of chemical change in an...
Gibbs Free Energy Calculations Chemistry Tutorial Calculating the Gibbs free energy (G) change for a chemical reaction using enthalpy (H), entropy (S) and temperature (T), tutorial with worked examples for chemistry students. The change in Gibbs free energy (ΔG) for a chemical reaction at constant temperature (T) and pressure can be calculated
Gibbs free energy - Wikipedia The classical Carnot heat engine. Book. Category. v. t. e. In thermodynamics, the Gibbs free energy (or Gibbs energy) is a thermodynamic potential that can be used to calculate the maximum reversible...
PDF 3.2 Gibbs Free Energy 3.2 Gibbs Free Energy. Josiah Willard Gibbs (1839-1903) was an American theoretical physi-cist, chemist, and mathematician. Use of Gibbs energy curves to construct a binary phase diagram the liquid and solid states. Source: Ref 3.3 as published in Ref 3.2.
Helmholtz and Gibbs Free Energies The Gibbs free energy G is defined by. The internal energy U might be thought of as the energy required to create a system in the absence of changes in temperature or volume. But as discussed in defining enthalpy, an additional amount of work PV must be done if the system is created from a very...
Gibbs Free Energy - Chemistry | Socratic The Gibbs Free Energy (delta G) is equal to the enthalpy (delta H) minus the temperature in Kelvin times the entropy (delta S). This serves as a measurement Gibbs free energy is no longer included on the UK A level syllabus. It includes both an enthalpy term (#DeltaH#), and entropy term (#DeltaS#).
Free energy | Endergonic vs exergonic reactions... | Khan Academy The Gibbs free energy change (ΔG) and how it's related to reaction spontaneity and equilibrium.
Gibbs (Free) Energy - Chemistry LibreTexts Gibbs free energy, denoted G , combines enthalpy and entropy into a single value. The change in free energy, ΔG , is equal to the sum of the enthalpy plus the product of the temperature and …
PDF The gibbs free energy behind the phase diagram of... MS15a, Gibbs Free Energy and Phase Diagrams 11/00. Note: the molar gibbs free energy of a pure element is often give the symbol µAo, as it is equivalent to the chemical potential of the pure element. As a function of composition...
Gibbs Free Energy Gibbs Free Energy is used to determine whether a reaction is favored or disfavored. It is given by the equation Even if a reaction has a positive ΔG, it can be driven by an external source of energy, such as an electric current, photons of light, or by coupling to a spontaneous reaction.
Gibbs Free Energy Formula : Structure, Preparations and Properties Gibbs Free Energy Formula- In thermodynamics, the Gibbs free energy or IUPAC name Gibbs energy or Gibbs function or free enthalpy is the thermodynamic potential. We use it to calculate the maximum reversible work that a thermodynamic system performs at constant pressure and...
Structural Biochemistry/Enzyme/Gibbs free energy graph - Wikibooks... The Gibbs free energy graph shows whether or not a reaction is spontaneous-- whether it is exergonic or endergonic. ΔG is the change in free energy. Generally, all reactions want to go to a lower energy state, thus a negative change is favored.
















0 Response to "40 gibbs free energy diagram"
Post a Comment