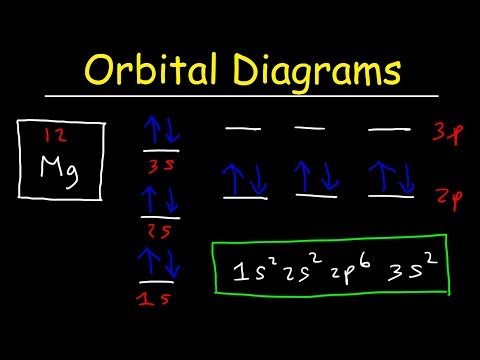
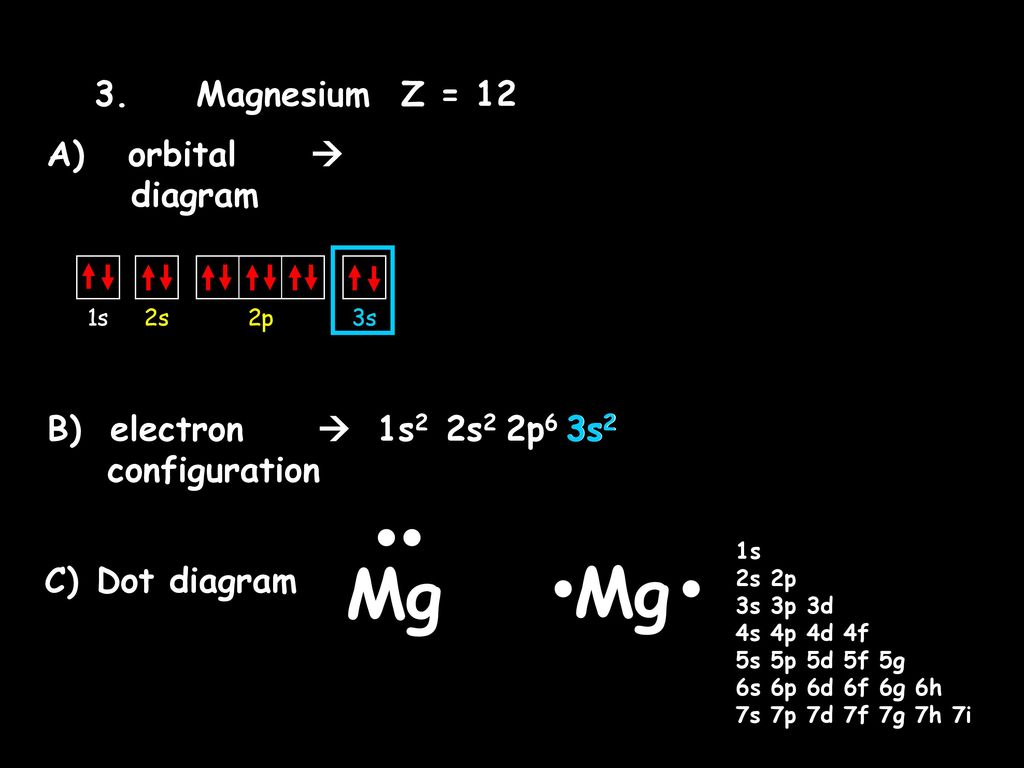
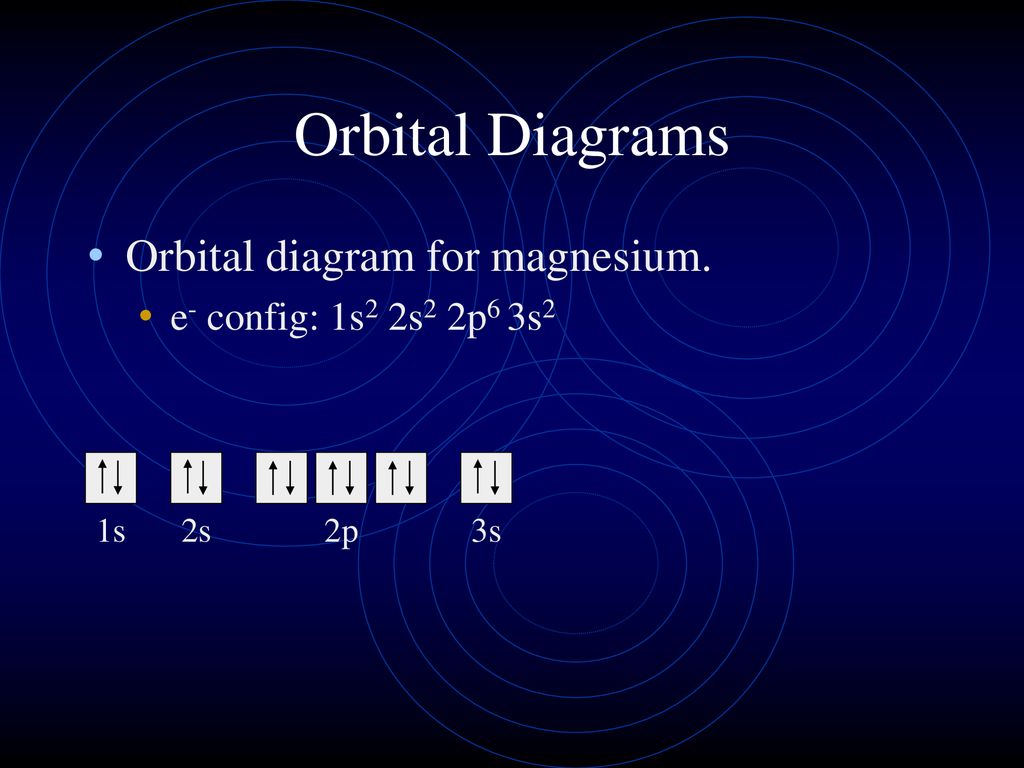
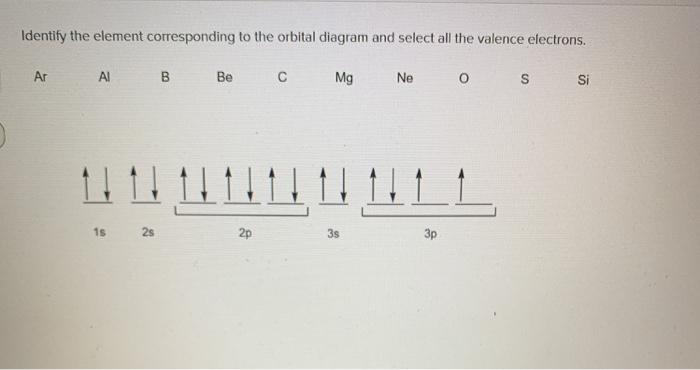
38 orbital diagram for magnesium
Is N2 Paramagnetic? - QuestionAnswer.io Step 2: The molecular orbital diagram for Li 2 ... Magnesium. Sodium. Which of the following is paramagnetic Cl2O? Paramagnetic species in the following is ClO2 only. - Cl2O has no unpaired electrons in it, so it is diamagnetic in nature. - ClO2 has one unpaired electron in it after bonding with 2 oxygen atoms, so it is paramagnetic in nature. What is the electron dot configuration for magnesium ... What is the electron dot configuration for magnesium? The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the remaining two electrons in the 3s. Therefore the Magnesium electron configuration will be 1s22s22p63s2. What is the dot diagram of magnesium oxide?
Valence Electrons Does Magnesium - Summarized by Plex.page ... Magnesium is found in group 2 of the periodic table's group 2. Magnesium has a melting point of 923 K and a boiling point of 1363 K. Magnesium also aids in the body's functions. Magnesium Valence Electrons Dot Diagram is shown as the one shell can only hold two electrons, shell two can hold eight electrons, and the third shell can hold eight ...

Orbital diagram for magnesium
what is the orbital diagram of magnesium chloride ... English. Secondary School. What is the orbital diagram of magnesium chloride? plus. Add answer + 5 pts. report flag outlined. Electron Configuration for Phosphorus (P) - UMD In writing the electron configuration for Phosphorus the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Phosphorous go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. Magnesium(Mg) electron configuration and orbital diagram Orbital Diagram for Magnesium (Mg) Electron configuration of magnesium ion(Mg 2+) Ground state electron configuration of magnesium is 1s 2 2s 2 2p 6 3s 2. After the electron configuration, the last shell of the magnesium atom has two electrons. In this case, the valency and valence electrons of magnesium are 2. The elements that have 1, 2, or 3 electrons in the last shell donate the electrons in the last shell during bond formation.
Orbital diagram for magnesium. Oxygen - Wikipedia Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds.Oxygen is Earth's most abundant element, and after hydrogen and helium, it is the third-most abundant element in the universe. Tellurium electron configuration: Clear your doubt ... Tellurium electron configuration. It has an atomic number of 52. To find out the electron configuration of an atom, we first need to know the total number of electrons. So here as this element has 52 atomic numbers, it means the element has 52 electrons. Once we know the number of electrons, we can start arranging the electrons in the orbitals. terpconnect.umd.edu › configurationMagnesiumElectron Configuration for Magnesium (Mg) Since 1s can only hold two electrons the next 2 electrons for magnesium go in the 2s orbital. The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the remaining two electrons in the 3s. Therefore the Magnesium electron configuration will be 1s 2 2s 2 2p 6 3s 2. Non-ionic Chemical Reactions - Michigan State University A molecular orbital diagram of ethene is created by combining the twelve atomic orbitals associated with four hydrogen atoms and two sp 2 hybridized carbons to give twelve molecular orbitals. Six of these molecular orbitals (five sigma & one pi-orbital) are bonding, and are occupied by the twelve available valence shell electrons.
Nitrogen Bohr Model - How to draw Bohr diagram for ... The Bohr Model of Nitrogen(N) has a nucleus that contains 7 neutrons and 7 protons. This nucleus is surrounded by two-electron shells named K-shell and L-shell. The outermost shell in the Bohr diagram of Nitrogen contains 5 electrons that also called valence electrons. magnesium is an electron donor - GKCWPC magnesium is an electron donor. Reducing agents "reduce" (or, are "oxidized" by) oxidizing agents. A requirement of the electron donor is that essential amounts monocyclopentadienyl titaniumtrichloride and a magnesium compound like and magnesium dichloride is dissolved in it. Magnesium has low ionization enthalpy that is it easily donates electron. Periodic Table - Periodic Table Are you searching for Calcium Electron Configuration (Ca) with Orbital Diagram? Every person should learn about the chemical Orbital Diagram, electronic configuration, atomic number, atomic mass, molecules, etc. It will not only be necessary for chemistry subjects, but it is also essential for general knowledge. What Is The Electron Configuration Of Magnesium The electron configuration for magnesium is 1s 2 2s 2 2p 6 3s 2 . How do you write electronic configuration? The symbols used for writing the electron configuration start with the shell number (n) followed by the type of orbital and finally the superscript indicates how many electrons are in the orbital.
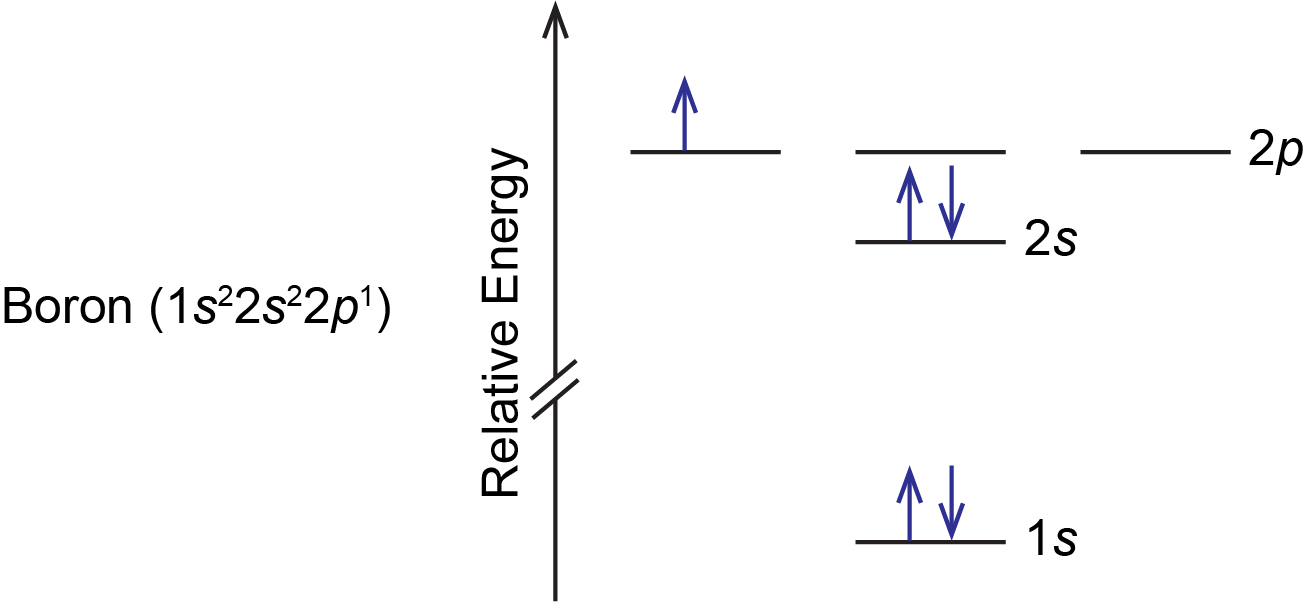
Orbital Diagram For Calcium (Ca) | Calcium Electron ... The 6 electrons will go to the 2p orbital, and the next 2 electrons will place with 3s orbital, and now we have only 8 electrons in which 6 electrons will go with 3p orbital, and the last 2 electrons will be with 4s orbital. So, we have Calcium Electron Configuration is: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s². So, the configuration helps to know the ... 39 complete an orbital diagram for boron. An orbital diagram is similar What is the orbital diagram for. For example, write the electron configuration of scandium, Sc: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 1. So for scandium the 1 st and 2 nd electron must be in 1s orbital, the 3 rd and 4 th in the 2s, the 5 th through 10 th in the 2p orbitals, etc. 6/14/ Ch 8 4/18 Correct Part B Complete ... The Electron Configurations of Atoms - Chemistry at Illinois Boron (atomic number 5) has five electrons. Four electrons fill both the 1s and 2s orbitals. The fifth electron is added to a 2p orbital, the sublevel next higher in energy (Figure 5.9). The electron configuration of boron is: B: 1s 2 2s 2 2p 1. Table 5.2 shows the electron configurations of the elements with atomic numbers 1 through 18. Electron configuration for Molybdenum (element 42 ... Orbital diagram. Molybdenum electron configuration ← Electronic configurations of elements . Mo (Molybdenum) is an element with position number 42 in the periodic table. Located in the V period. Melting point: 2617 ℃. Density: 10.28 g/cm 3. The order of filling the orbitals with electrons in the Mo atom is an exception to the rule. ...
Nitrogen(N) electron configuration and orbital diagram Nitrogen(N) is the 7th element in the periodic table and its symbol is ‘N’. This article gives an idea about the electron configuration of nitrogen and orbital diagram, period and groups, valency and valence electrons of nitrogen, bond formation, compound formation, application of different principles.Hopefully, after reading this article you will know in detail about this.
Konfigurasi Elektron, Diagram Orbital, Contoh Soal, dan ... Konfigurasi Elektron, Diagram Orbital, Contoh Soal, dan Pembahasannya Gurubagi.com. Atom terdiri atas inti dan elektron yang beredar mengitarinya menurut lintasannya masing-masing. Untuk mengetahui bagaimana lintasan elektron tersebut, maka dapat kita melihat penyebaran elektron dalam kulit-kulit elektron melalui konfigurasi elektron.
how many unpaired electrons are in the boron atom ... By Hund's rule, the electron configuration of carbon, which is 1s 2 2s 2 2p 2, is understood to correspond to the orbital diagram shown in c. Experimentally, it is found that the ground state of a neutral carbon atom does indeed contain two unpaired electrons.
Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13: Orbital diagram of Aluminum (Al) 14: Orbital diagram of Silicon (Si) 15: Orbital diagram of Phosphorus ...
Electronic Configuration of Magnesium Cation- Mg²+ - Bob ... Magnesium cation (Mg2+) is, as stated above, is a cationic /less stable derivative of Mg formed after It gives up two of its valency or outer electrons from its electron shell or orbital. Electronic Configuration of Mg2+ tells us how many electrons are arranged in each shell of the Mg2+ atom and its shape. Mg2+ has an electronic configuration ...
Sodium Bohr Model - How to draw Bohr diagram for Sodium(Na ... The Bohr Model of Sodium(Na) has a nucleus that contains 12 neutrons and 11 protons. This nucleus is surrounded by three-electron shells named K-shell, L-shell, and M-shell. The outermost shell in the Bohr diagram of Sodium contains only 1 electron that also called valence electron.
what is the electron configuration of boron - Lisbdnet.com Therefore the Magnesium electron configuration will be 1s22s22p63s2. The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. What is e9 class configuration? Electronic configuration is defined as the distribution of electrons into the orbitals of an atom.
What is the orbital diagram for magnesium? - Quora Aug 20, 2015 — What is the electron configuration of magnesium using SPDF and an IT orbital diagram? Magnesium: Atomic number = 12. Electronic configuration is 1s2 2s2 2p6 ...
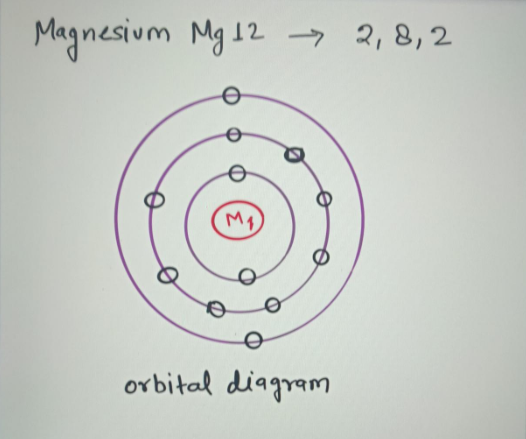
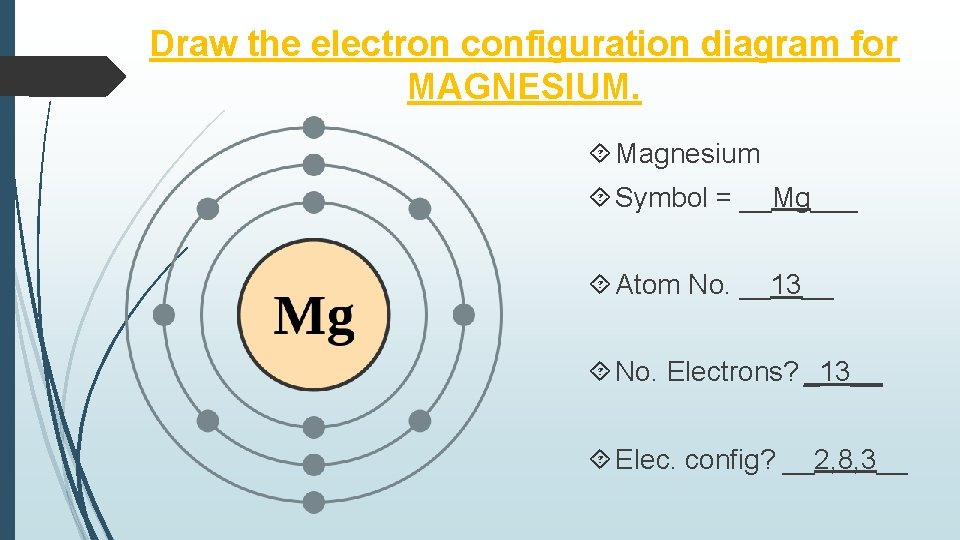
Magnesium (Mg) - Periodic Table (Element Information & More) Here is the table showing the capacity of orbits to hold electrons. Number of electrons in shells. Thus, 1st shell can hold 2 electrons. 2nd shell can hold 8 electrons. 3rd shell can hold 18 electrons. 4th shell can hold 32 electrons. Now the atomic number of Magnesium (Mg) is 12. Hence the magnesium element has electrons arrangement 2, 8, 2.
molecular orbital theory diagrams - Allergy Link molecular orbital theory diagrams Fluorimetry of selenium in body fluids after digestion with nitric acid, magnesium nitrate hexahydrate, and hydrochloric acid. February 18, 2022 by Jeremy.
Electron Configuration For Magnesium - ViralListClub.com Electron configuration of Magnesium is Ne 3s2. Magnesium has 2 valence outer-shell electrons and will lose both to fulfill the octet rule. The p orbital can hold up to six electrons. Since 1s can only hold two electrons the next 2 electrons for magnesium go in the 2s orbital. The nex six electrons will go in the 2p orbital.
D. Write the orbital diagram of the following elements ... D. Write the orbital diagram of the following elements. Abbreviate using a oblees. 1. Carbon 2. Magnesium 3. Selenium 4. Antimoni 5. Mercury
Chemical Bonding Dalal Simplified ICSE Chemistry Class-10 ... Chemical Bonding Dalal Simplified ICSE Chemistry Class-10. By PANDEY TUTORIAL Last updated Jul 30, 2021. (1) An ionic equation. (2) Electron dot structural diagram. (3) Atomic or orbital structural diagram the formation of the following. (a) Sodium chloride. (b) Calcium oxide.
The electron configuration for magnesium is 1s^2 2s^2 2p^6 ... The electron configuration for magnesium is 1s2 2s2 2p6 3s2. What is the distribution of electrons in the electron shells of a magnesium atom? · The first two ...1 answer · Top answer: Answer: The number of electrons for the Mg atom are 12 electrons. The electron configuration of magnesium is, Mg (Z= 12) = 1s2 2s2 2p6 3s2 The first ...
What Is The Electron Configuration For Magnesium Is 1S^2 ... Magnesium Cation Mg2+ Magnesium cation (Mg2+) is, as declared above, is a cationic /less secure derivative the Mg created after It gives up 2 of the valency or external electrons from its electron covering or orbital.Electronic construction of Mg2+ tells united state how countless electrons are arranged in each shell of the Mg2+ atom and its shape.
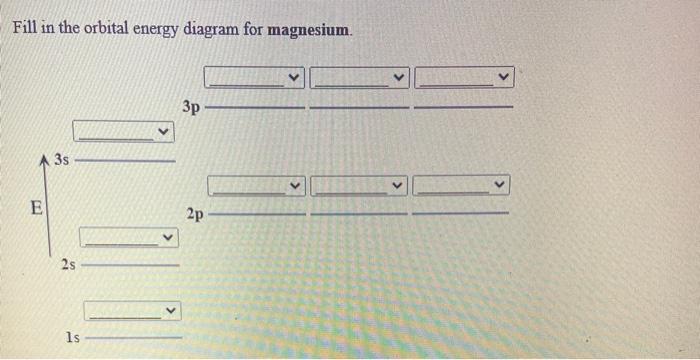
study.com › academy › answerWrite the full orbital diagram for magnesium. | Study.com Write the full orbital diagram for magnesium. Orbital Diagram: The subatomic particle that occupies most of the volume of an atom is known as the electron. The region of space wherein electrons ...
Draw the orbital diagram of the following elements 1 ... Draw the orbital diagram of the following elements 1. Magnesium 2. Fluorine - Brainly.ph. Draw the orbital diagram of the following elements 1. Magnesium 2. Fluorine . See what the community says and unlock a badge.
topblogtenz.com › magnesium-orbital-diagramMagnesium Orbital diagram, Electron configuration, and ... The orbital diagram for Magnesium is drawn with 4 orbitals. The orbitals are 1s, 2s, 2p, and 3s. The Magnesium orbital diagram contains 2 electrons in 1s orbital, 2 electrons in 2s orbital, the six electrons in 2p orbital, and the remaining two electrons in 3s orbital.
Orbital Rotation Jeopardy Template True or False: This is the orbital diagram for Magnesium? False. 300. What are the orbitals letters? s,p,d,and f. 300. what is this element . manganese. 300. what is this element? strontium . 300. draw the orbital notation for Titanium (Ti) 400. True or False: This is the orbital notation for Calcium.
Which element has a higher 3rd ionization energy, Al or Mg ... Nov 27, 2015 · In magnesium's case, however, the third electron would come from the second energy level, more specifically from a 2p-orbital. Since this third electron is located closer to the nucleus for magnesium than for aluminium, you can expect the third ionization energy to be higher in magnesium's case.
Filling of Electrons in Orbitals: Definition, Properties ... Filing of Electrons in Orbitals: The representation of electrons in various shells, subshells, and orbitals is known as electronic configuration. The arrangement of electrons is of great importance in chemistry. In chemistry, electron configurations can be utilised to justify the chemical properties of elements.
How to draw electron configuration diagrams, magnesium ... 1. For an s orbital, draw a circle for a p orbital, draw a figure eight for a d orbital, draw a four-leafed clover for an f orbital, see below. This results in a diagram with two filled 2p orbitals and one half-filled p orbital. For the purposes of the answer, I'll simply provide the electron-configuration notation, which you can then translate to.
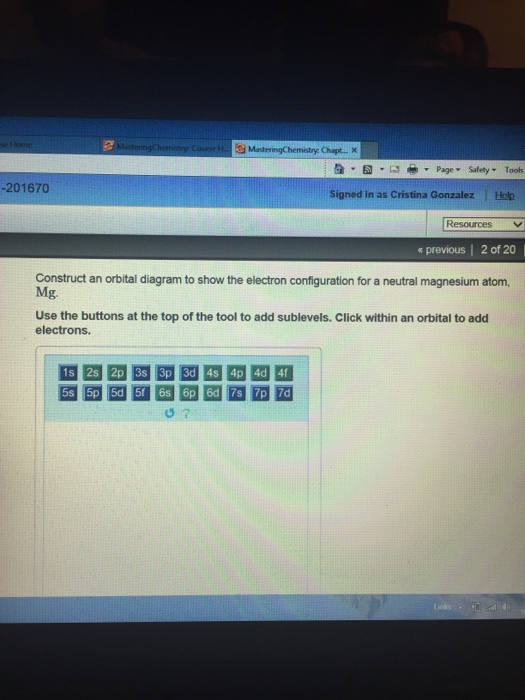
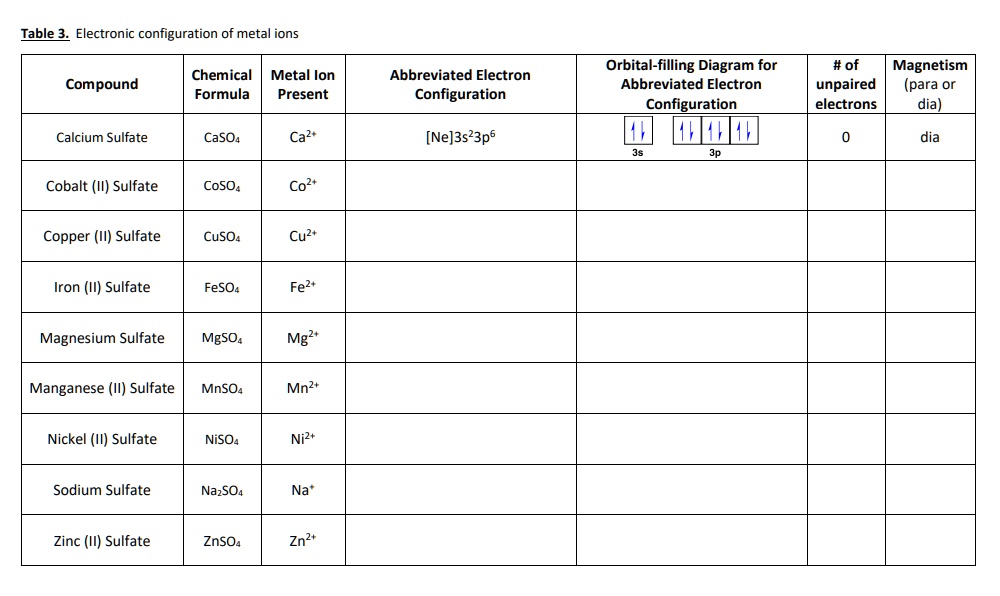
(Get Answer) - Cation Anion Formula Name Magnesium ... Cation Anion Formula Name Magnesium bicarbonate SrCl2 Selection A Manganese II) chlorate 2+ Co PO4 3- Selection B Cu2CO3 Selection C Construct the molecular orbital diagram for N2 and then identify the bond order Bond order 0.5 O 1.5 O 2.5 2s 2s Click within the blue boxes to add electrons.
Selina Concise Chapter 4 Atomic Structure and Chemical ... Draw the orbital diagram of ion and state the number of three fundamental particles present in it. Solution. ... orbital diagram of the Magnesium chloride (b) orbital diagram of the Nitrogen (c) orbital diagram of the Methane (d) orbital diagram of the Hydrogen chloride. 13. State the type of bonding in the following molecules.
Magnesium(Mg) electron configuration and orbital diagram Orbital Diagram for Magnesium (Mg) Electron configuration of magnesium ion(Mg 2+) Ground state electron configuration of magnesium is 1s 2 2s 2 2p 6 3s 2. After the electron configuration, the last shell of the magnesium atom has two electrons. In this case, the valency and valence electrons of magnesium are 2. The elements that have 1, 2, or 3 electrons in the last shell donate the electrons in the last shell during bond formation.
Electron Configuration for Phosphorus (P) - UMD In writing the electron configuration for Phosphorus the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Phosphorous go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons.
what is the orbital diagram of magnesium chloride ... English. Secondary School. What is the orbital diagram of magnesium chloride? plus. Add answer + 5 pts. report flag outlined.


![Energy range covered by the bands of MgO [20] and the ...](https://www.researchgate.net/profile/John-Larese/publication/228522494/figure/fig5/AS:667684391895047@1536199772710/Energy-range-covered-by-the-bands-of-MgO-20-and-the-molecular-orbitals-of-NO-and-NO-2.png)




















0 Response to "38 orbital diagram for magnesium"
Post a Comment