42 magnesium fluoride lewis dot diagram
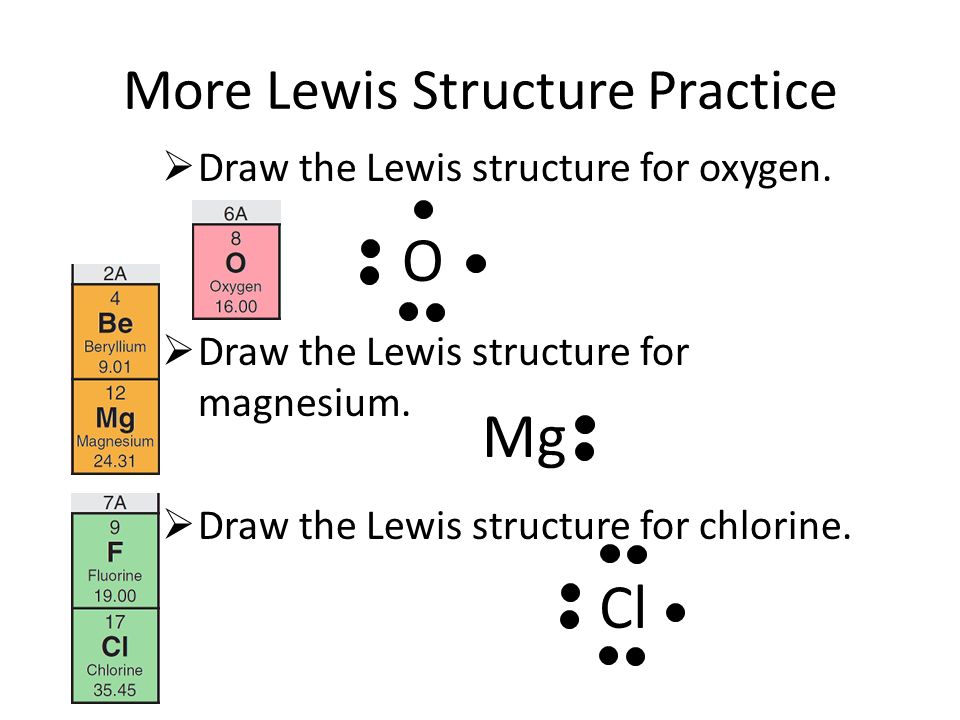
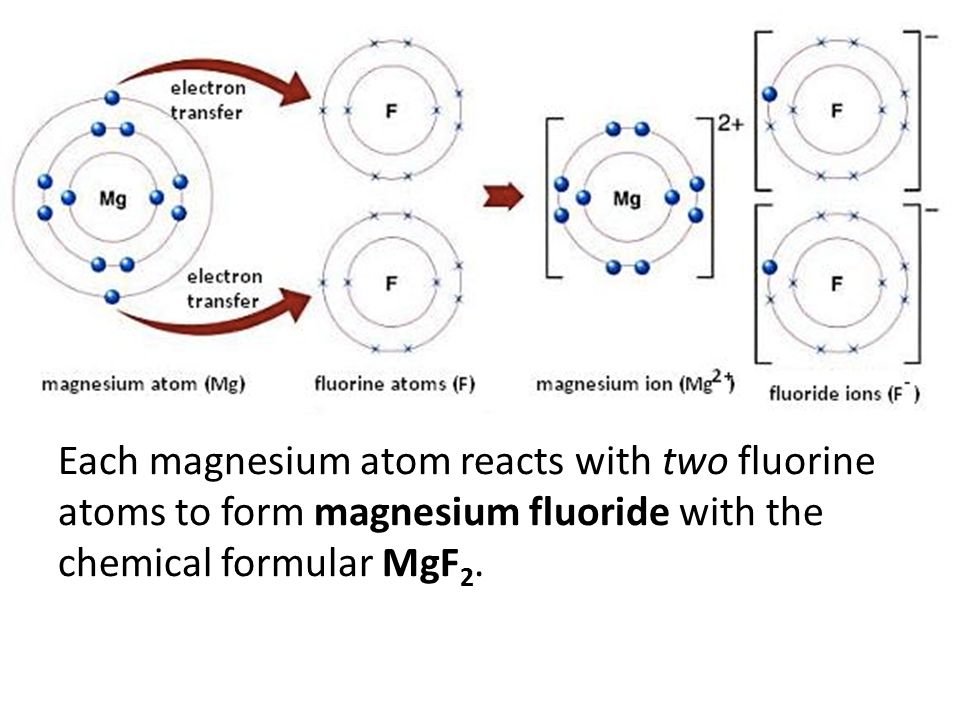
PDF Lewis Dot Structures of Atoms and Ions 1. Using Lewis dot diagrams, show how some number of atoms of magnesium and atoms of fluorine can transfer electrons to form ions of each element with stable octets. Be sure to include the resulting charges of the ions. Circle the part of your diagram that shows the formation of one formula unit of magnesium fluoride. DOCX Lewis Dot Structures Worksheet - Mr. Walsh's Class Draw the Lewis Dot Structure. Notes: Scientists use. Lewis Dot Structures. to show the valance electrons of an element as dots. Since bonding involves the valance shell electrons only, it is only necessary to illustrate those outer electrons. ... Determining the formula for Magnesium Fluoride? Identify the charges = Mg. 2+ F. 1. Cross the ...
Draw the electron dot diagram of the following compounds. 1 ... Click here to get an answer to your question ✍️ Draw the electron dot diagram of the following compounds. 1. NaF 2. MgF2 [Hint: Atomic No.1 answer · Top answer: Answr has image solution available for this question

Magnesium fluoride lewis dot diagram
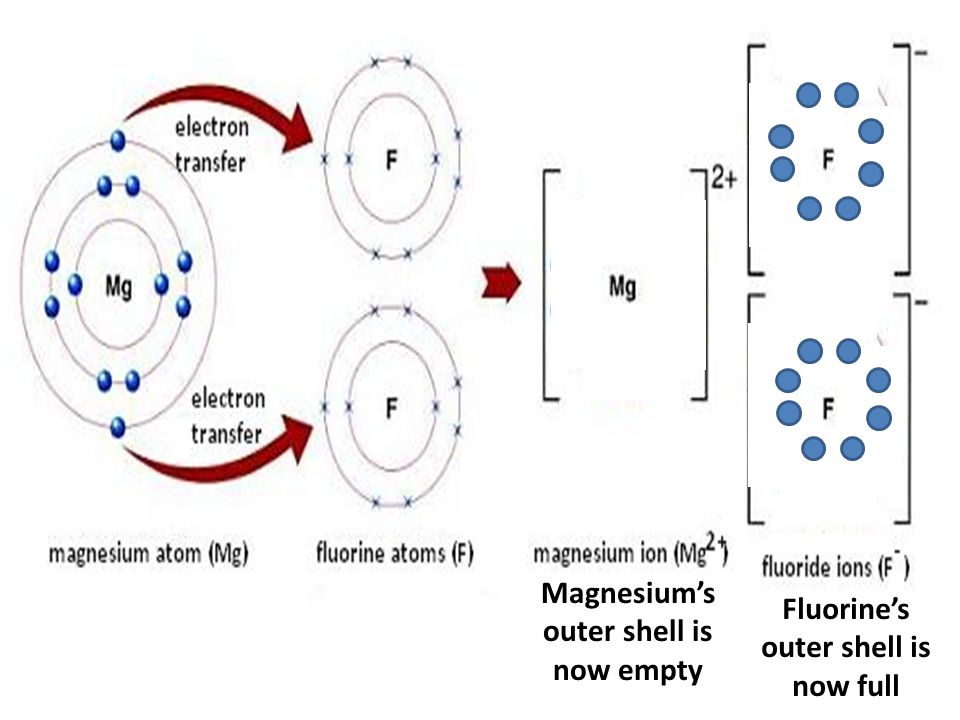
DOCX Lewis Dot Diagrams - birdvilleschools.net Lewis Dot Diagrams Ionic compounds Lithium fluoride, LiF Aluminum chloride, AlCl3 Cesium nitride, Cs3N Barium arsenide, Ba3As2 Magnesium phosphide, Mg3P2 Calcium chloride, CaCl2 Magnesium bromide, MgBr2 Rubidium sulfide, Rb2S Potassium oxide, K2O Beryllium oxide, BeO Francium sulfide, Fr2S Sodium chloride, NaCl What is the correct lewis electron-dot structure for ... - Socratic Each Florine starts with seven electrons around the atom, combining with the Magnesium atom give the Florine eight electrons around each Florine atom. This eight electrons are found in four pairs. shown in the diagram as a pair on top a pair on the left a pair on the bottom and a pair on the left. Answer link OneClass: What is the correct Lewis electron-dot structure ... Get the detailed answer: What is the correct Lewis electron-dot structure for the compound magnesium fluoride? Get the detailed answer: What is the correct Lewis electron-dot structure for the compound magnesium fluoride? 🏷️ LIMITED TIME OFFER: GET 20% OFF GRADE+ YEARLY SUBSCRIPTION → ...
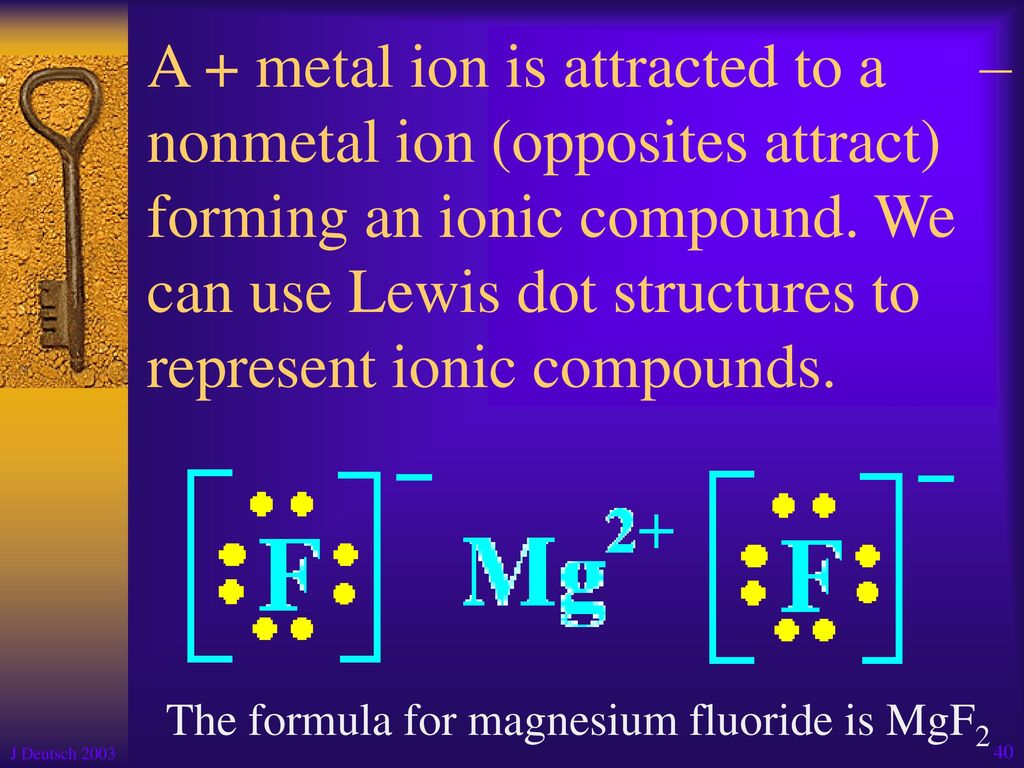
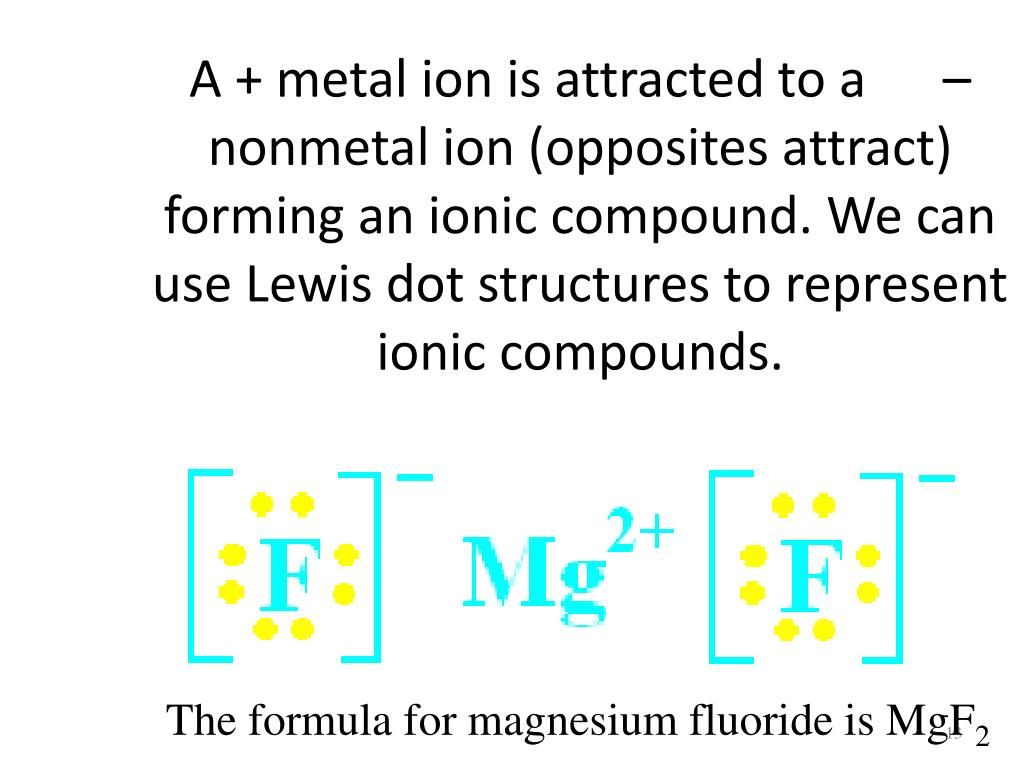
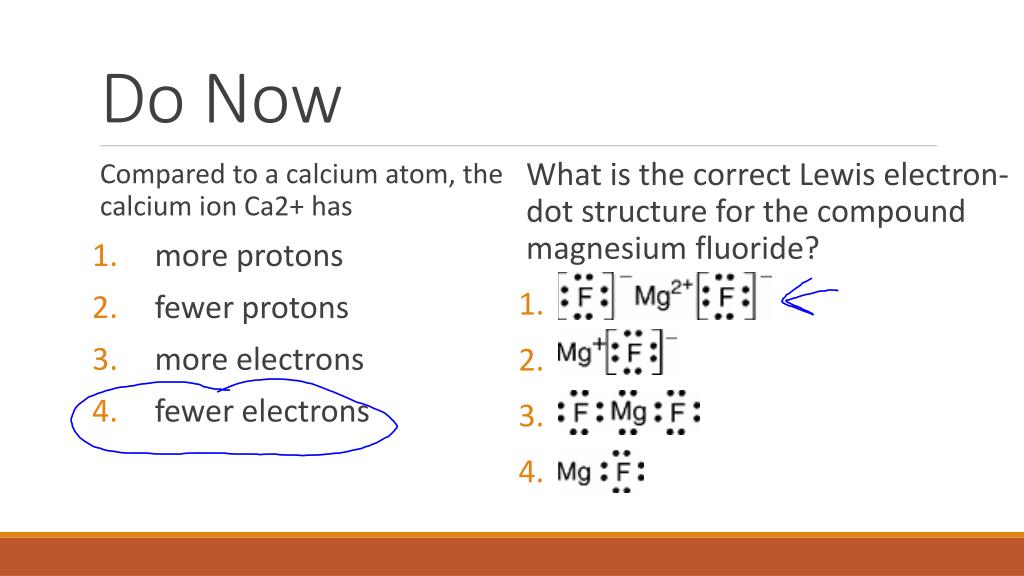
Magnesium fluoride lewis dot diagram. What is the Lewis dot structure of $\text{Mg}{{\text{F}}_{\text{2}}} $\text{Mg}{{\text{F}}_{\text{2}}}$ is an ionic compound made up of magnesium cation and fluoride anion. To draw the electron dot or Lewis diagram of this ...1 answer · Top answer: Hint: To draw the electron dot diagram of $\text{Mg}{{\text{F}}_{\text{2}}}$, we need to find the number of valence electrons that take part in bonding ... PDF Magnesium iodine lewis dot structure The lewis dot structure for sulfur is an s with 6 dots which stand for its six valence electrons. The lewis dot structure for magnesium is an mg with 2 dots which stand for its two valence electrons. Speed up invoice data extraction 6x by using ai. Lewis dot diagram for magnesium is actually amongst pictures libraries inside our highest ... What Is The Correct Lewis Electron-Dot Structure For The ... Magnesium is element #12. To draw the Lewis electron dot diagram we picture in our minds the symbol for Mg in a box with all of its core electrons (i.e., 1s 2 2s 2 2p 6 ). Then we place the valence electrons around the sides of the box with each side representing an orbital in the outermost energy level. What does Mg2+ mean? magnesium ion (Mg2+) What is the correct lewis electron-dot structure for ... - Brainly.in Apr 11, 2019 — Each Florine starts with seven electrons around the atom, combining with the Magnesium atom give the Florine eight electrons around each Florine ...2 answers · 13 votes: Magnesium has two electrons on its outer shell Each of the electrons will be shared with a ...
What is the Lewis Structure for magnesium fluoride? - Answers Magnesium fluoride doesn't have a Lewis structure. Lewis structures are only used to show covalent bonds and magnesium fluoride forms an ionic bond. As a general rule of thumb, my chem teacher... Magnesium Fluoride Lewis Dot Diagram Magnesium Fluoride Lewis Dot Diagram Magnesium fluoride is prepared from magnesium oxide with sources of hydrogen fluoride such as ammonium bifluoride. Magnesium has two electrons on its outer shell Each of the electrons will be shared with a Florine atom. This results in a compound MgF2. potassium fluoride lewis dot structure - ofcs.org The Lewis Structure (electron dot diagram) of each ion is used to construct the Examples Lithium fluoride, LiF Lithium atom loses one electron to form the. Like other sources of the fluoride ion, F−, KF is poisonous, although lethal doses approach gram levels for humans. The pages include calendars for each class, notes, homeworks, worksheets ... PDF Dot and cross diagram of calcium fluoride Dot and cross diagram of calcium fluoride Learn Chemistry 11 with Eva & Nicole: Electronic Structure Ionic Bonding Quiz 2 ProProfs Quiz How the ionic bond forms in Sodium Sulfide (Na2S) YouTube 15 2: Electrons are Transferred in Ionic Compounds 34 Chemistry Worksheet Lewis Dot Structures Answer Key ShowMe Orbital diagram of calcium Learn Chemistry 11 with Eva & Nicole:
Lewis Structure For Aluminum Fluoride - Novocom.top magnesium dot fluoride cross diagram lewis valence electrons fluorine drawing formula compound ionic shell mgf diagrams chemistry showing properties . fluoride molybdenum osmium hexafluoride iridium rhenium rhodium svg fluorine compounds wikipedia wikimedia commons names . Which of the following is the Lewis dot structure for the ... 1. Draw the Lewis dot structure (electron dot) for each of the following atoms: a. Se b. P c. C d. I. 2. Draw the Lewis structure for each of the following ions: a. N3-b. Ga3+ c. S2-d. O2-3. Classify the following compounds as ionic (I) or covalent (C): a. FeCl2 b. NO2 c. CO d. AlP. 4. Give the Lewis structure for each of the following: a. NF5 ... What is the electron dot configuration for magnesium ... One can draw the 3-dimensional structure of an atom once they have the Lewis Structure of an atom. The 3-dimensional geometrical structure of ammonium, NH4+ is referred to as Tetrahedral. Nitrogen, having 5 valence shell electrons, along with 4 from Hydrogen, should have had 9 electrons. Magnesium Fluoride Lewis Dot Diagram - schematron.org Which Lewis electron-dot diagram represents an atom in the ground state for a the correct Lewis electron-dot structure for the compound magnesium fluoride?. A Lewis electron-dot symbol is a symbol in which the electrons in the valence at the transfer of electrons from magnesium to fluorine to form magnesium fluoride.
a Explain the formation of magnesium fluoride using class ... - The Lewis dot structure of the magnesium fluoride is as: Now coming to the next part of the question; - Coordinate covalent bonds are those bonds in which the one atom gives its both the electrons from the shared pair. In other words, we can also say that the both electrons are derived from the same atom.
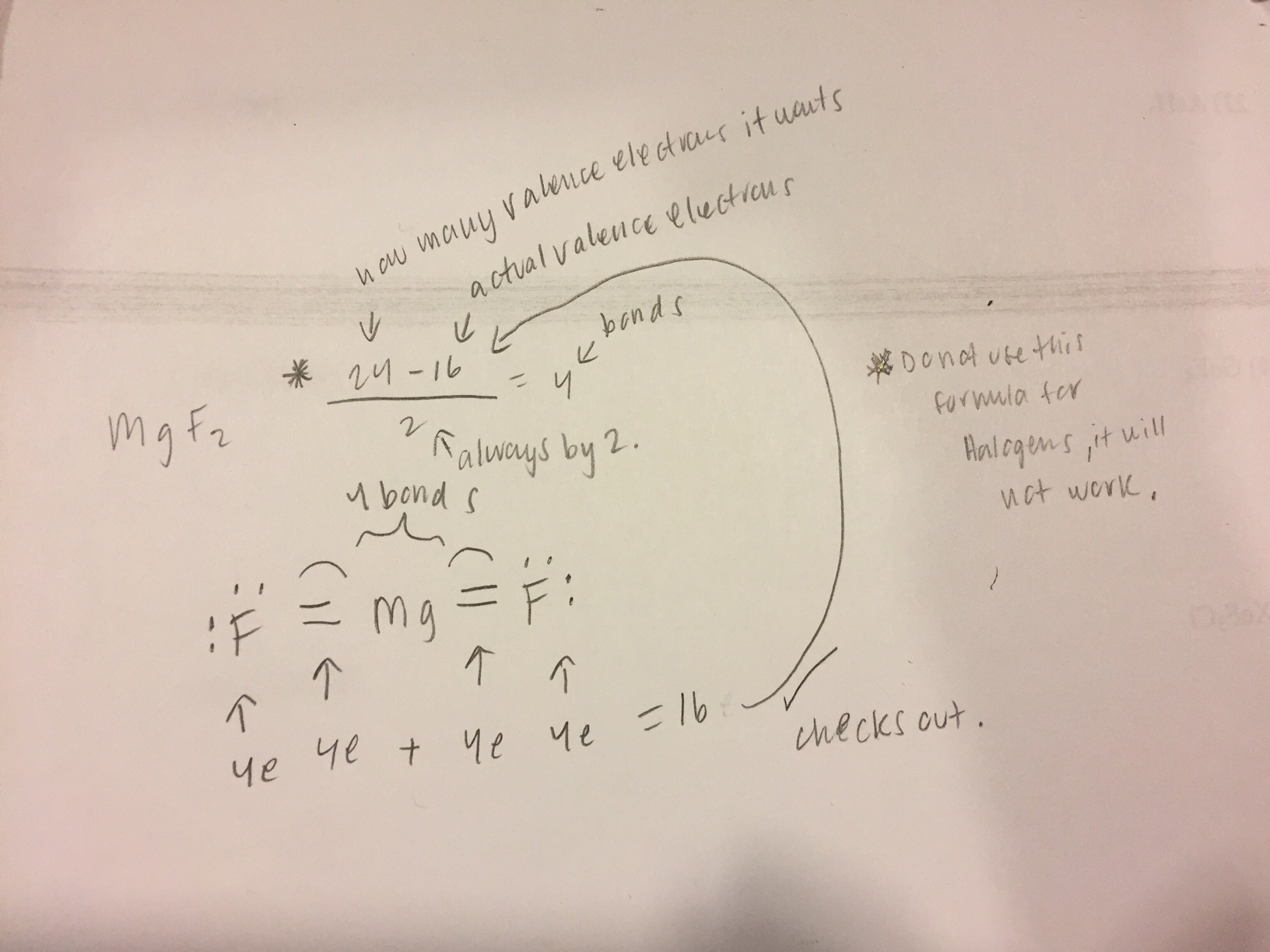
MgF2 Lewis Structure, Geometry, Hybridization, and Polarity It is so because the Lewis structure is drawn for such molecules where sharing of valence electrons takes place, which is the case with magnesium fluoride. Also called electron dot structures, the Lewis diagrams help with studying the reasons behind the atoms within a molecule achieving the most stable position to remain unexcited.
PPT PowerPoint Presentation - Chemical BONDING Chemical BONDING IONIC Lewis Dot Diagrams Sodium Chloride This is the finished Lewis Dot Structure [Na]+1 [ Cl ]-1 How did we get here? Practice Dot diagrams & formulas Lithium fluoride Magnesium oxide Calcium chloride Potassium hydride Drawing molecules using Lewis Dot Structures Remember: atoms are sharing e- to complete their outer shell!
⚗️What is the correct lewis electron-dot structure for the ... The correct electron dot structure that represents the Magnesium fluoride molecule has been C.. Lewis electron dot structure has been used for the representation of the valence electrons and the bonded electrons in the compound.The electrons have been represented with the dot, thereby the structure has been termed to be the dot structure.. Magnesium has been consisted of 12 electrons with 2 ...
potassium fluoride lewis dot structure - ICC A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The bifluoride on heating yields potassium fluoride: Platinum or heat resistant plastic containers are often used for these operations.
Which Lewis Dot Diagram Represents A Fluoride Ion Which Lewis Dot Diagram Represents A Fluoride Ion. Learn how metals react to form ionic compounds and how this effects their properties with BBC Bitesize GCSE Chemistry. Representing negative ions. The following It gains an electron from another atom in reactions, forming a fluoride ion, F -. A fluoride ion has the same electronic structure as ...
Lewis Dot Diagram For Magnesium Fluoride - schematron.org Which Lewis electron-dot diagram represents an atom in the ground state for a the correct Lewis electron-dot structure for the compound magnesium fluoride?.Dec 18, · Best Answer: Magnesium has 2 valence electrons and Fluorine has 7 valence electrons in order for the 2 elements to combine, you need 1 Mg atom and 2 F atoms The Mg gives one ...
Draw the Lewis Structure for MgF2 (magnesium fluoride ... One magnesium atom loses two electrons, to become a +2 ion (cation).Two fluorine atoms gain one electron each to become two -1 ions (anions).These are held t...
How to draw MgF2 Lewis Structure? - Science Education and ... Step-1: MgF2 Lewis Structure. To calculate the valence electron of each atom in MgF2, look for its periodic group from the periodic table. The alkaline earth metal and halogen families, which are the second and 17th groups in the periodic table, are both made up of magnesium and fluorine atoms. In their outermost shells, magnesium and fluorine ...
lewis dot structures of ions.pdf - Lewis Dot Structures of ... B. Lewis dot structure for a chloride ion is Chlorine needs an additional electron to attain the stable noble gas configuration of 8 valence electrons. Since chlorine is a nonmetal, it has relatively high values for electronegativity and ionization energy. This means that it will gain electrons until it achieves a stable octet. When chlorine becomes an ion we add one more dot to the atom's ...
What is the correct lewis electron-dot structure for the ... Lewis-dot structure : It shows the bonding between the atoms of a molecule and it also shows the unpaired electrons present in the molecule. The valence electrons are represented by 'dot'. The given molecule is, phosphorus trihydride. As we know that phosphorous has '5' valence electrons and hydrogen has '1' valence electron.
Magnesium And Phosphorus Lewis Dot Structure - Novocom.top Magnesium And Phosphorus Lewis Dot Structure, Dot Cross Diagram Magnesium Fluoride YouTube, Step 4A: Place one lone pair on the center P atom to reach, What are valence electrons give example, phosphorus electrons Images Frompo 1
OneClass: What is the correct Lewis electron-dot structure ... Get the detailed answer: What is the correct Lewis electron-dot structure for the compound magnesium fluoride? Get the detailed answer: What is the correct Lewis electron-dot structure for the compound magnesium fluoride? 🏷️ LIMITED TIME OFFER: GET 20% OFF GRADE+ YEARLY SUBSCRIPTION → ...
What is the correct lewis electron-dot structure for ... - Socratic Each Florine starts with seven electrons around the atom, combining with the Magnesium atom give the Florine eight electrons around each Florine atom. This eight electrons are found in four pairs. shown in the diagram as a pair on top a pair on the left a pair on the bottom and a pair on the left. Answer link
DOCX Lewis Dot Diagrams - birdvilleschools.net Lewis Dot Diagrams Ionic compounds Lithium fluoride, LiF Aluminum chloride, AlCl3 Cesium nitride, Cs3N Barium arsenide, Ba3As2 Magnesium phosphide, Mg3P2 Calcium chloride, CaCl2 Magnesium bromide, MgBr2 Rubidium sulfide, Rb2S Potassium oxide, K2O Beryllium oxide, BeO Francium sulfide, Fr2S Sodium chloride, NaCl

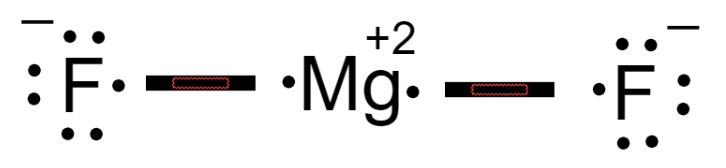


![Solved What is the correct Lewis structure for MgF2? [Mg]2+ ...](https://media.cheggcdn.com/media/6c2/6c2e92a2-d75e-431f-aa94-7280f101dece/phpU8nfhQ)


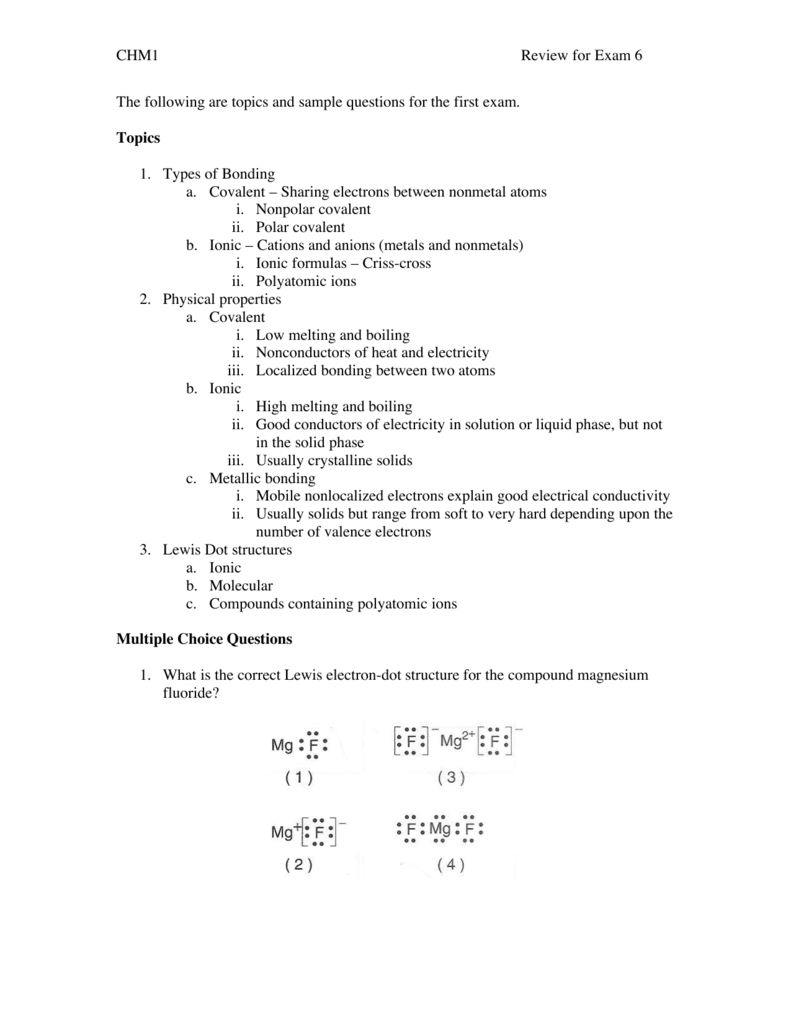
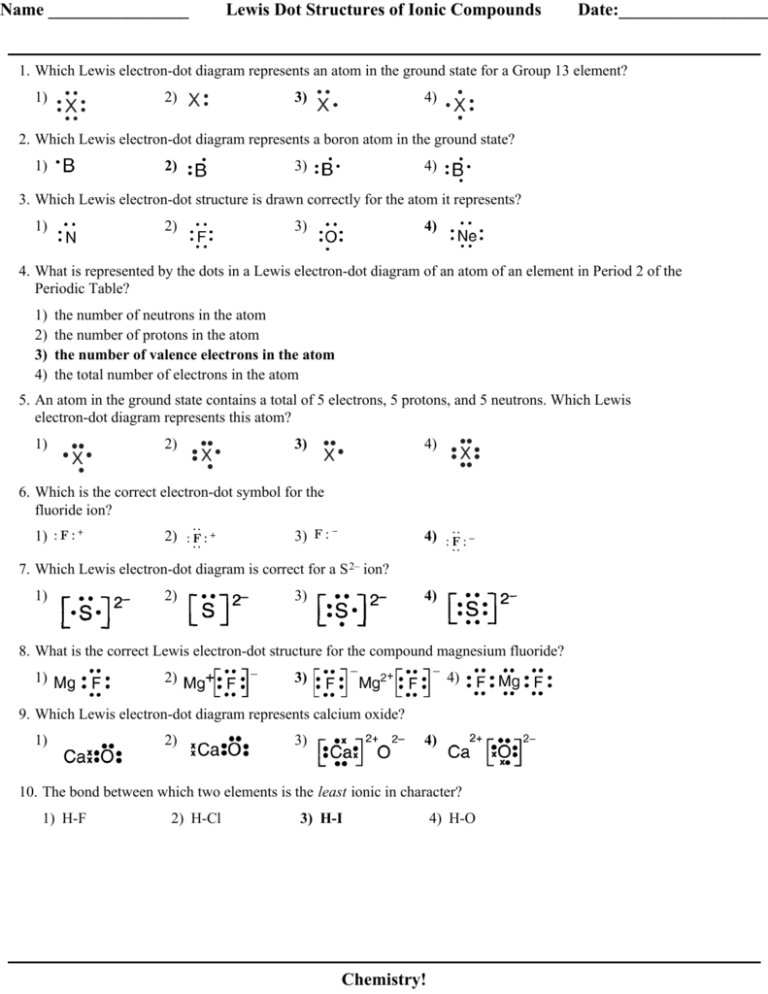





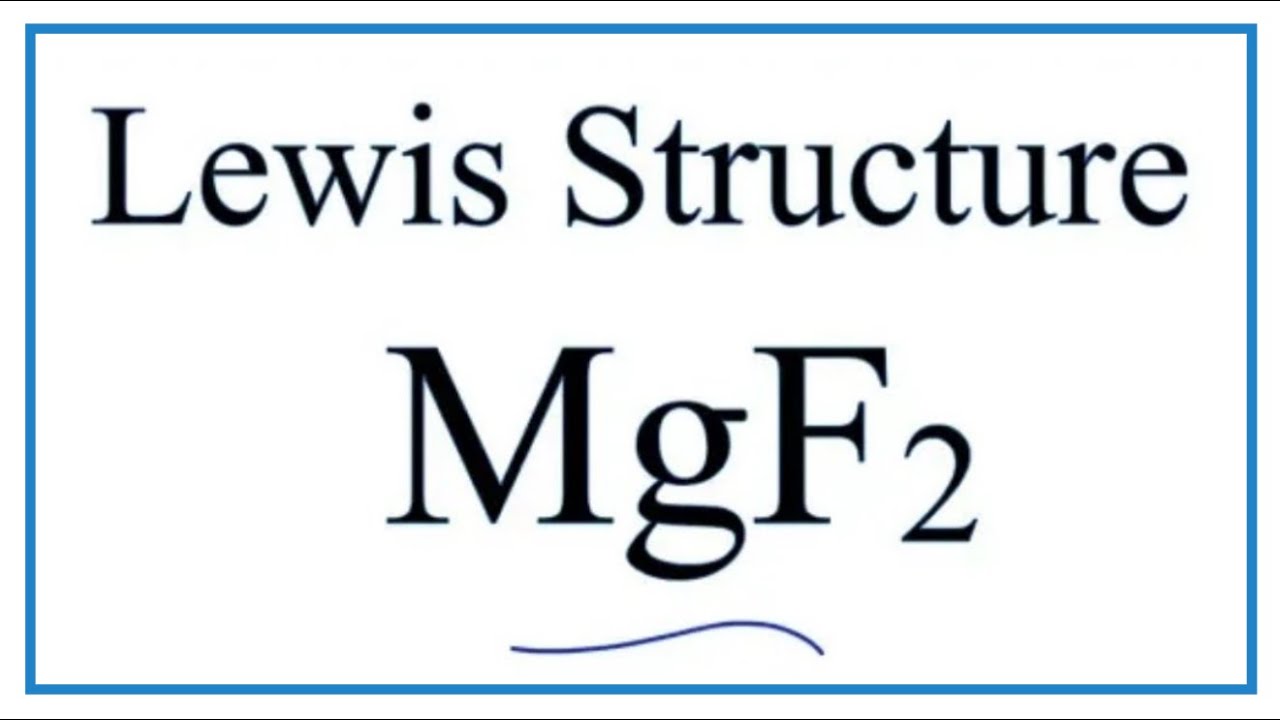






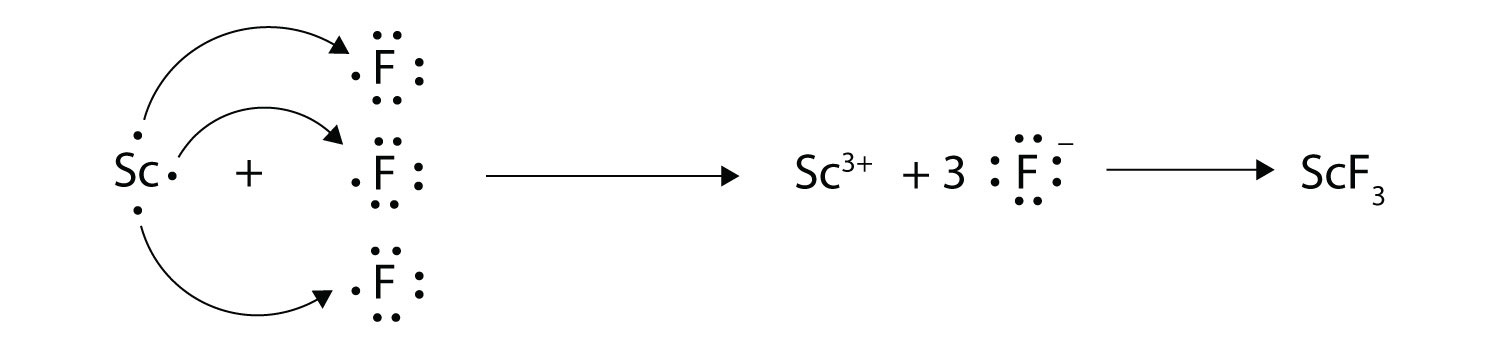





0 Response to "42 magnesium fluoride lewis dot diagram"
Post a Comment