41 write the orbital diagram of carbon before sp3 hybridization
Write the orbital diagram of carbon before {eq}\rm sp^3 {/eq} hybridization. Hybridization: Hybridization is a hypothetical concept that describes the contribution of each orbital before bonding ... Write orbital diagrams to represent the electron configuration of carbon before sp3 hybridization.
Write the orbital diagram of carbon before sp hybridization. Use the buttons at the top of the tool to add orbitals. Click within the orbital to add electrons. Question: Write the orbital diagram of carbon before sp hybridization. Use the buttons at the top of the tool to add orbitals. Click within the orbital to add electrons.
Write the orbital diagram of carbon before sp3 hybridization
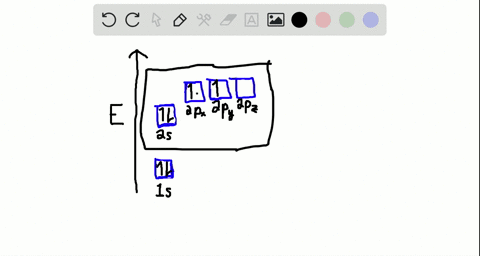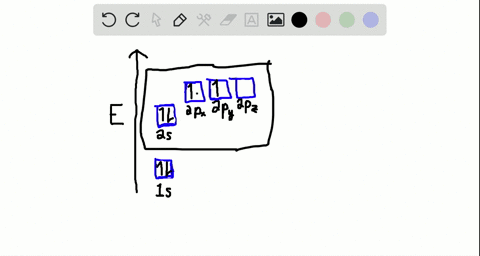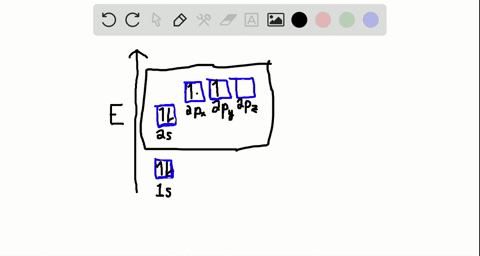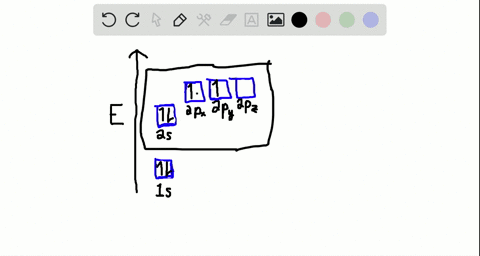
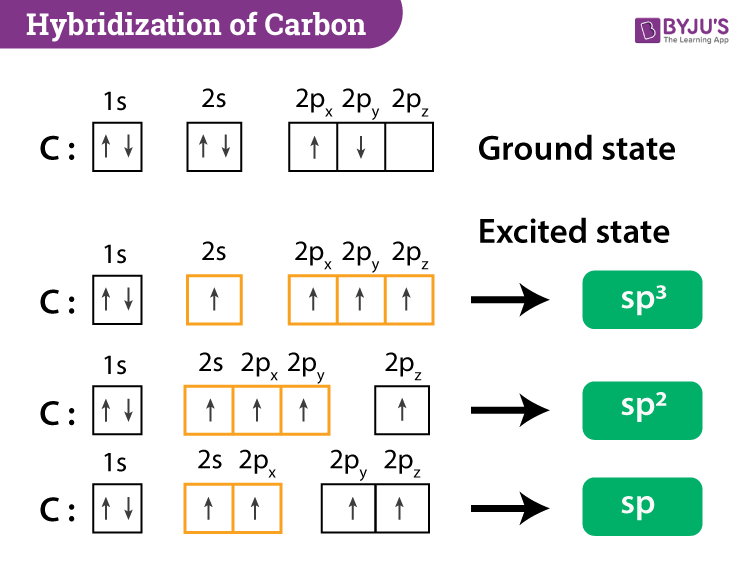
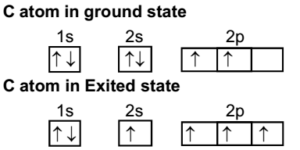
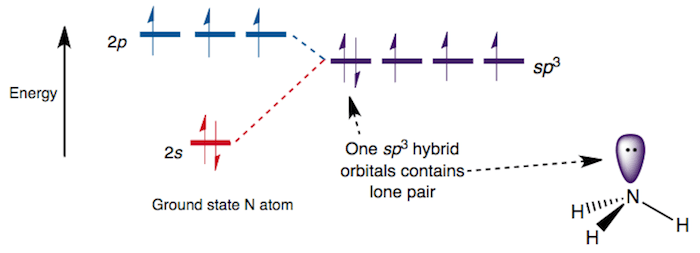
Write the orbital diagram of carbon before sp3 hybridization. Consider the electron configuration of a carbon atom. The angle between them is 180o making co 2 a linear molecule as predicted by vespr. The hydrogens 1s orbital bonds with well each of the hydrogens 1s orbital bonds with each of the carbons sp3 orbitals. Write orbital diagrams (boxes with arrows in them) to represent the electron configuration of carbon and then show sp3, sp2 and sp hybridization. Write a hybridization and bonding scheme for each molecule. Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 10.6 and 10.7 (in your book). Carbon is the sixth element with a total of 6 electrons. In writing the electron configuration for carbon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for C goes in the 2s orbital. The remaining two electrons will go in the 2p orbital. Therefore the C electron configuration will be ...
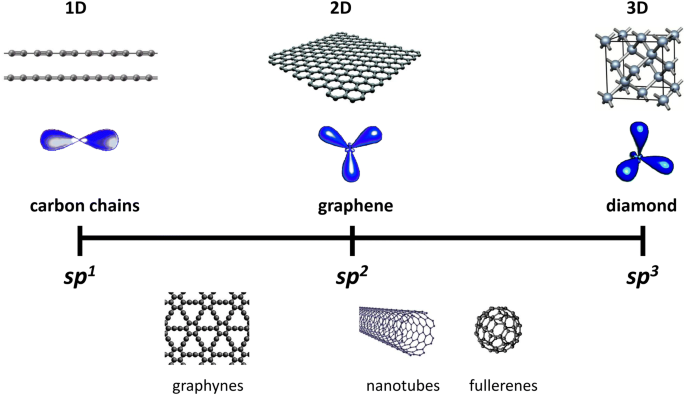
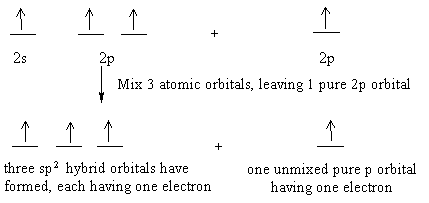
Write the orbital diagram of carbon before sp3 hybridization. Orbital hybridization is essentially a process of mixing orbitals together and spitting out new ones that are all identical in "symmetry" and. Answer to write orbital diagrams to represent the electron configuration of carbon before sp3 hybridization. The atomic number of carbon is 6, which is also the number of The orbital diagram shows how ... The new orbitals formed are called sp 3 hybrid orbitals. These are directed towards the four corners of a regular tetrahedron and make an angle of 109°28' with one another. The angle between the sp3 hybrid orbitals is 109.28 0; Each sp 3 hybrid orbital has 25% s character and 75% p character. Example of sp 3 hybridization: ethane (C 2 H 6 ... Orbital hybridization is essentially a process of mixing orbitals together and spitting out new ones that are all identical in "symmetry" and "composition" to the orbital(s) from the other, incoming atom(s).. You can read more about #sp^3# hybridization here.The qualitative energies turn out to be the following:. with #sp^3# hybridized orbitals of #25%# #s# character and #75%# #p# character. sp Hybridization: When Carbon is bound to two other atoms with the help of two double bonds or one single and one triple bond. Example: Hybridization of CO2. sp2 Hybridization: When carbon atom bonding takes place between 1 s-orbital with two p orbitals then the formation of two single bonds and one double bond between three atoms takes place. Example: Hybridization of graphite
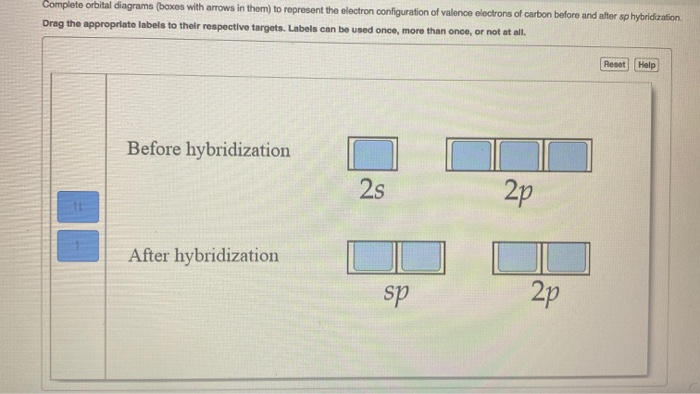
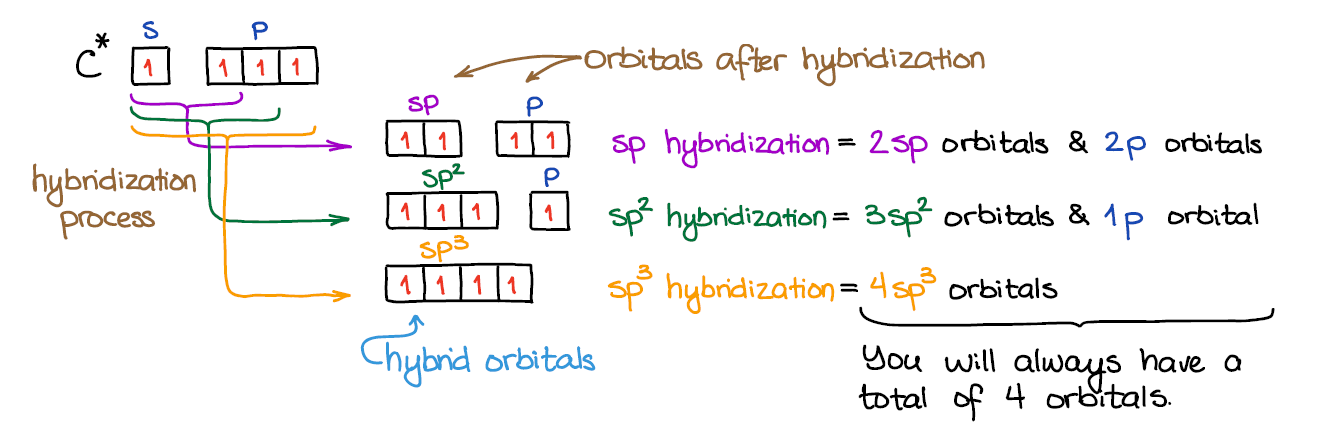
Write The Orbital Diagram Of Carbon Before Sp3. Click Images to Large View Write The Orbital Diagram Of Carbon Before Sp3. Sp3 Sp2 And Sp Hybridization In Organic Chemistry With. ... ازاي تعرف نوع التهجين في الذرة Hybridization Sp Sp2. Write orbital diagrams (boxes with arrows in them) to represent the electron configuration of carbon before and after sp3 hybridization. FREE Expert Solution. Before hybridization, we have: 100% (117 ratings) Problem Details. Carbon has an electron configuration of 1s^2 2s^2 2p^2. During sp hybridization, one s and one p orbital of carbon combine to form two sp hybrid orbitals. The molecular, sp 3 orbitals are arranged in a tetrahedron, with bond angles of 109.5 o. Each of the 1s orbitals of H will overlap with one of these hybrid orbitals to give the predicted tetrahedral geometry and shape of methane, CH 4. Hybridization also changes the energy levels of the orbitals. The 2s orbital of carbon is lower in energy than the 2p orbitals, since it is more penetrating.
Write the orbital diagram of carbon before sp3 hybridization. Orbital hybridization is essentially a process of mixing orbitals together and spitting out new ones that are all identical in symmetry and. Hybridization of atomic orbitals explained s sp sp2 and sp3 organic chemistry sp3 sp2 and sp hybridization when two atomic 1s orbitals combine ... Part L Identify the hybridization of all interior atoms for the molecule CH3 SH according. what does the atomic orbital diagram of carbon look like orbital hybridization is essentially a because the 2p orbital what does the atomic orbital diagram of carbon look like before sp 3. Hybridization of tetravalent carbon. In fact the latter picture is more accurate the lone pair of electrons on an amide nitrogen are not localized in an sp3 orbital rather. Nov 10, 2018 · So no, the atom doesn't have to get excited to 1s2 2s1 2p3 before In the case of sp3 hybridization, say in methane, the carbon s orbital. Orbital hybridization is essentially a process of mixing orbitals together and spitting out new ones that are all identical in "symmetry" and. The atomic number of carbon is 6, which is also the number of The orbital diagram shows how the electrons are arranged within each sublevel. rule, each orbital must contain one electron each with the same spin, before. The atomic number of carbon is 6, which is also the number of positively charged protons its atomic nuclei. If the atom is neutral, it will have the same number of negatively charged electrons. Its electron configuration is "1s"^2"2s"^2"2p"^2". The orbital diagram shows how the electrons are arranged within each sublevel. The maximum number of electrons allowed in an orbital is 2, each with ...
sp hybridization. In sp hybridization, one s orbital and one p orbital hybridize to form two sp orbitals, each consisting of 50% s character and 50% p character. This type of hybridization is required whenever an atom is surrounded by two groups of electrons. Created by Jay. This is the currently selected item.

Oneclass Write Orbital Diagrams To Represent The Electron Configuration Of Carbon Before Sp3 Hybridi
Answer to write orbital diagrams to represent the electron configuration of carbon before sp3 hybridization. Write the orbital diagram of carbon before sp3 hybridization. Please just explain what the orbital looks like. So no, the atom doesn't have to get excited to 1s2 2s1 2p3 before In the case of sp3 hybridization, say in methane, the carbon s orbital.
Write The Orbital Diagram Of Carbon Before Sp3 Hybridization. Write The Orbital Diagram Of Carbon Before Sp3 Hybridization - Diagram ... Write The Orbital Diagram Of Carbon Before Sp3 Hybridization - General ...
The hybrid orbitals are placed in a triangular arrangement with 120° angles between bonds. Example: Hybridization of graphite. 3. sp 3 Hybridization. When the carbon atom is bonded to four other atoms the hybridization is said to be sp 3 type. Here 1 s orbital and 3 p orbitals in the same shell of an atom combine to form four new equivalent ...
Solved Draw Orbital Diagrams Boxes With Arrows In Them To Represent The Electron Configurations Of Carbon Before And After Sp Hybridization
Write the orbital diagram to represent the electron configuration of carbon before sp hybridization. Orbital diagram notation Condensed electron configurations are quite simple.
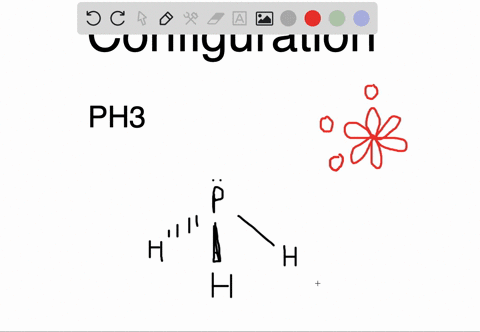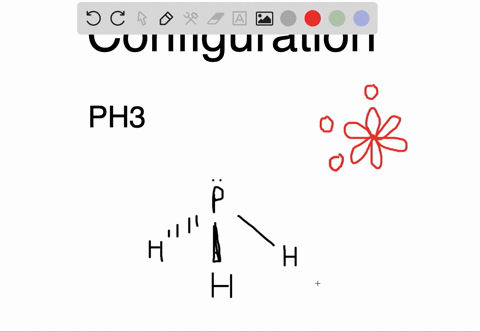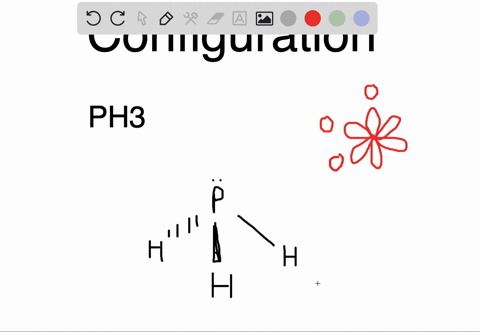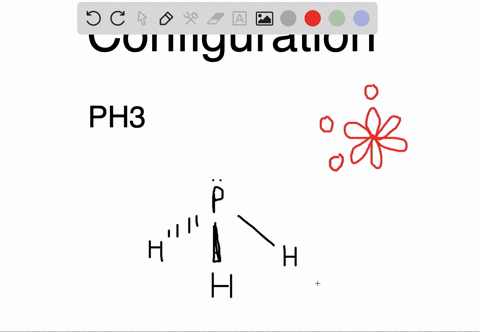
The carbon atoms form a σ sp 2 sp 2 bond with each other by using sp 2 hybrid orbitals. The p orbitals are equal in energy and said to be degenerate. Write the orbital diagram of carbon before sp 3 hybridization. Write the orbital diagram of carbon before sp hybridization. How do you write the orbital diagram for carbon.
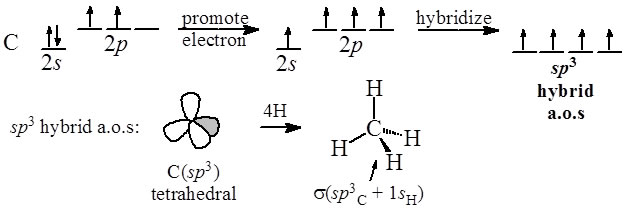
Worksheet 14 - Hybridization The hybridized molecular orbitals have different shapes and energy levels than the atomic orbitals. The number of molecular orbitals created by hybridization depends on the number of atomic orbitals that are mixed to form them. In forming sp3 hybridized orbitals, four atomic orbitals are mixed, one s and three p.
An sp3 orbital looks something like this. This is a hybridized sp3 orbital. Hybrid just means a combination of two things. A hybrid car is a combination of gas and electric. A hybridized orbital is a combination of s and p. Hybridized sp3 orbitals are the orbitals when carbon bonds with things like hydrogen or really when it bonds with anything.
Write The Orbital Diagram Of Carbon Before Sp3. ... P4s3 Hybridization. 좋은 습관 Hybrid Orbitals Sp3 Sp2 Sp Carbon. Sp3 Sp2 And Sp Hybridization In Organic Chemistry With. Explain Me About Sp Sp2 Sp3 Hybridisation With Examples. ... 29 Write The Orbital Diagram Of Carbon Before Sp3.

17 Sketch The Orbital Diagram Valence Shell Only Of Carbon Before And After Hybridization Of Its Homeworklib
Answer (1 of 3): Hybridisation -C≡C- In a carbon-carbon triple bond ,one is sigma bond and the other two bonds are pi bonds (e g. HC≡CH). * Here carbon undergoes sp hybridization and the two sp hybridized orbitals formed involve in sigma bond formation. * Bonds in H-C≡C-H 1 sigma sp-sp ; 2 s...
the sp3 hybrid orbitals on C with the 1s atomic orbitals from the hydrogen atoms. The carbon-carbon sigma bond is formed from overlap of an sp3 hybrid orbital on each C atom. Ethanol, C2H6O has 2(4) + 6(1) + 6 = 20 e − The two C atoms and the O atom are sp3 hybridized. All bonds are formed from overlap with these sp3 hybrid orbitals.

Take Methane And Use It As An Example Of All The Concepts Listed Here Orbital Hybridization Homeworklib
Carbon is the sixth element with a total of 6 electrons. In writing the electron configuration for carbon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for C goes in the 2s orbital. The remaining two electrons will go in the 2p orbital. Therefore the C electron configuration will be ...
Write orbital diagrams (boxes with arrows in them) to represent the electron configuration of carbon and then show sp3, sp2 and sp hybridization. Write a hybridization and bonding scheme for each molecule. Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 10.6 and 10.7 (in your book).
Write the orbital diagram of carbon before sp3 hybridization. Consider the electron configuration of a carbon atom. The angle between them is 180o making co 2 a linear molecule as predicted by vespr. The hydrogens 1s orbital bonds with well each of the hydrogens 1s orbital bonds with each of the carbons sp3 orbitals.
Solved Draw Orbital Diagrams Boxes With Arrows In Them To Represent The Electron Configurations Of Carbon Before And After Sp Hybridization
Solved Write Full Orbital Diagrams For He Drag The Appropriate Labels To Their Respective Targets Labels Can Be Used Once More Than Once Or Not Course Hero
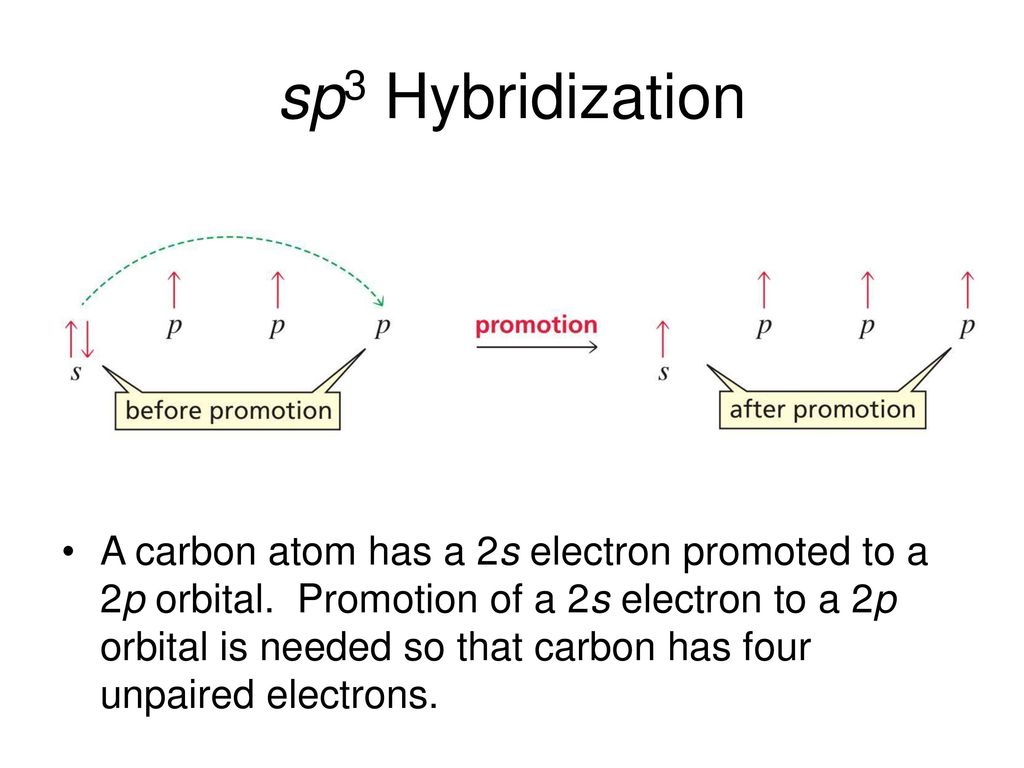
Lecture 2 Chemical Bonds Atomic Orbital Theory And Molecular Orbital Theory Dr A K M Shafiqul Islam Ppt Download

The Role Of Oxygenic Groups And Sp3 Carbon Hybridization In Activated Graphite Electrodes For Vanadium Redox Flow Batteries Hassan Chemsuschem Wiley Online Library




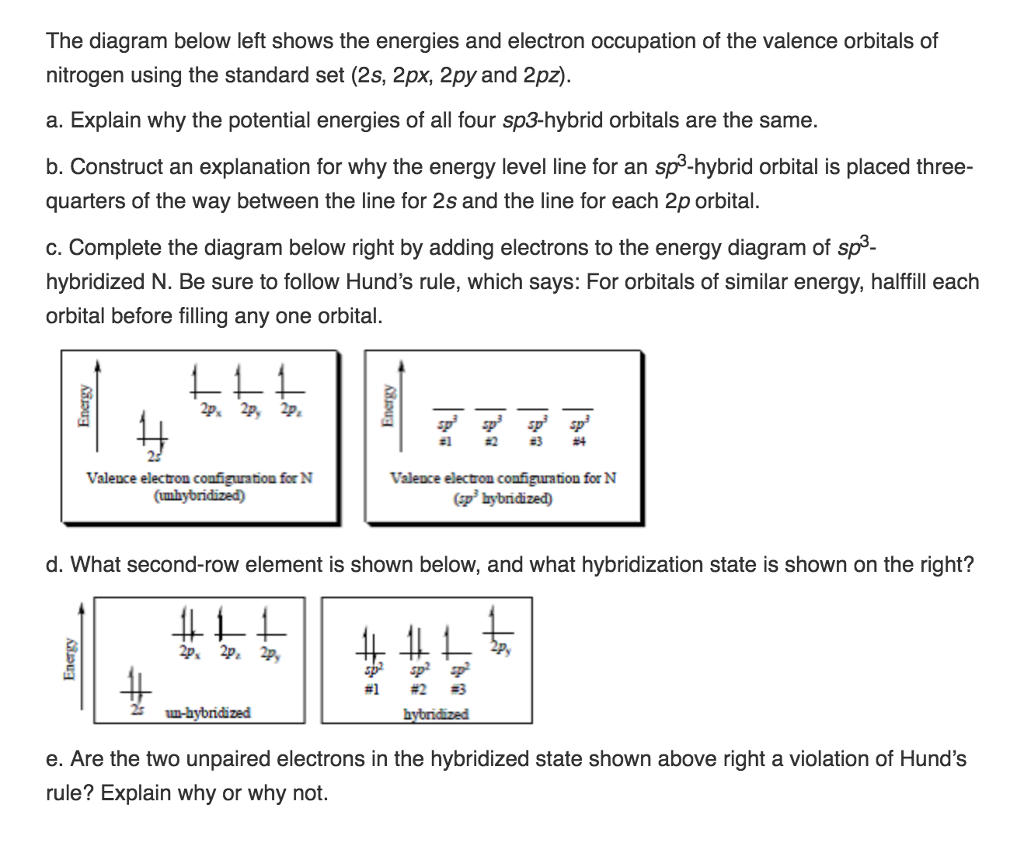





.jpg?revision=1&size=bestfit&width=601&height=192)


0 Response to "41 write the orbital diagram of carbon before sp3 hybridization"
Post a Comment